
The future of laboratory medicine in the era of precision medicine
Introduction
As a rule of thumb, laboratory medicine is an essential medical science which considerably contributes to the clinical decision making (1). Laboratory information is now used for screening, diagnosing, prognosticating and monitoring the vast majority of human diseases. In recent years, i.e., more specifically at the dawn of this century, medical science has undergone a substantial revolution, wherein the traditional approach to diagnosing and treating many human diseases has gradually evolved from a generalized conception of health and disease, as conventionally written in the medical textbooks, to an individualized approach entailing decisions and interventions tailored to the single patient according to individual responses or risk of disease. This model is now clearly translated into the concept of “personalized” or “precision” medicine (2). Although the route has already been tackled, the many opportunities for reworking our daily clinical practice to the new framework of precision medicine are challenged by a number of mounting variables and capricious scenarios, which may ultimately frustrate many expectations. Therefore, the aim of this article is to discuss some of the several changes which may ultimately drive or challenge the future of laboratory medicine in the era of precision medicine.
Drivers of changes in laboratory diagnostics
Healthcare reforms
There is little doubt that some ongoing healthcare reforms around the globe are the main factors contributing to drive the most essential changes. Due to the fact that the healthcare policies vary widely, the impact of in vitro diagnostics testing is also quite heterogeneous in both terms of organization, funding and reimbursement policies. There is now consolidated evidence that the gross domestic product (GDP) is the leading element influencing the local healthcare funding (3). The public and private (i.e., the so-called “out-of-pocket”) healthcare expenditure is dramatically different worldwide. For example, the medium cost of healthcare in the entire world is around 10% of the GDP, but the gap is rather wide, approximating 17.1% of the GDP in the United States, 11.5% in France, 11.3% in Germany, 9.2% in Italy and United Kingdom, 5.5% in China, but is as low as 4% in countries like Nigeria, Malaysia and Qatar (4). The Out-of-pocket health expenditure as percentage of total expenditure on health is also extremely different, ranging from 71.7% in Nigeria, 35.3% in Malaysia, 32.0% in China, 21.2% in Italy, 13.2% in Germany, 11.0% in the United States, 9.7% in the United Kingdom, 6.9% in Qatar and 6.3% in France (5) (Figure 1). Although it remains rather questionable whether or not a higher healthcare expenditure really translates into tangible health benefits (6), it is undeniable that a strict relationship exists with the cost of in vitro diagnostic testing. This has been convincingly demonstrated by recent analyses, showing that the cost of laboratory diagnostics in the United States approximated 2.4%, but is considerably lower (i.e., between 1.4–1.6%) in countries like Italy and Germany (7,8). Interestingly, these figures are not reflected by the common perception that many physicians have about the economic impact of laboratory diagnostics, since many of them still believe that in vitro diagnostics may erode more than 10% of the total healthcare resources (8), thus placing in vitro diagnostics testing in the unpleasant role of being considered a “high-consuming resources” industry.
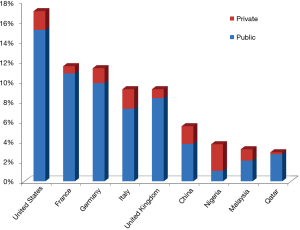
The impact of reforms on public and private healthcare expenditures has been quite recently reaffirmed after the presidential elections, in the United States. The Affordable Care Act, also known as “Obamacare”, has been established by former President Barak Obama some years ago and is still not completely realized (9). Due to the election of Donald J. Trump, the impact of this reform on public coverage of the resident population is likely to be considerably attenuated (10), thus potentially leaving as many as 11 million United States citizens without public healthcare coverage and reimbursement for many healthcare services, including laboratory tests. It is quite obvious that this would impact strongly the future of laboratory medicine industry in that country, especially on the private investment and research & development of in vitro diagnostic companies in this field.
Cost containment policies
A progressive contraction of healthcare funding is the most obvious consequence of the aforementioned scenario. The direct relationship existing between local healthcare expenditure and GDP, coupled with the huge economic crisis that is still plaguing many countries around the globe, make it very likely that cost-containment policies will grow exponentially in the foreseeable future. There are only two potential solutions for facing this contingency. The former entails a considerable reduction of number or type of tests that clinical laboratories may be able to perform. Considering that most tests that are (and will increasingly be) used in a personalized approach necessitate specific equipment, skilled personnel and expensive reagents, it is rather predictable that this emerging branch of in vitro diagnostic testing may be strongly impacted by cost-reduction policies. The use of biological treatments, especially those based on monoclonal antibodies against cell-surface structures expressed by cancer cells and lymphocytes, or against immunoglobulins (i.e., IgE) or cytokines such as tumor necrosis factor alpha (TNF-α) is becoming commonplace for treating of a kaleidoscope of human disorders. Genetic and epigenetic analysis is also prepotently emerging as a paradigm, not only for managing, but also for preventing, certain types of cancers (e.g., the assessment of breast cancer susceptibility gene—BRCA—for considering cancer risk-reducing surgery, such as bilateral prophylactic mastectomy or bilateral salpingo-oophorectomy) (11). The cost of these tests exceeds by several orders of magnitude the expenditure for other conventional laboratory examinations such as creatinine, glucose and potassium, among others. Will these costs be sustainable for most national healthcare systems in the future? We do not have a crystal ball, nor we can imagine how policymakers will revise the local criteria for reimbursement, but many doubts remain as to whether all these tests will be available to everybody, at least in public laboratories. Regardless of these general considerations, what clear clearly emerges from the current economic scenario is that we should be forced to rearrange our facilities “to do more with less” in order to complying with the ever increasing demand of personalized care.
Consolidation
The paradigm of “consolidation” is not new to the healthcare industry. Test duplication within laboratories insisting on the same geographical area has been a matter of debate for decades. Beside “stat” (i.e., urgent) tests, which should be made available in the shortest possible time for allowing timely diagnoses and treatments, no obvious reasons exist why the so-called routine tests should be carried out by many different laboratories located closely one to the other (12). Even so, the current models of consolidation not always follow reasonable criteria.
The archetype of consolidating activities in healthcare has been derived from the aviation industry. In 1955 the Delta Air Lines first established the so-called “hub-and-spoke” model, by consolidating the departures and arrivals of many long flights in its hub in Atlanta (Georgia, US) and creating major routes to connect peripheral airports (i.e., the “spokes”) in which long route flights had been suppressed. That allowed to optimize human and technical resources, generating considerable savings for the company and larger availability of intercontinental connections for the passengers. Nevertheless, the translation of this model to the healthcare industry cannot be straightforward and painless, since efficiency is just one of variables for evaluating healthcare organization, and is not certainly the most important (13). A suitable model of reorganization in laboratory diagnostics requires that both sustainability and effectiveness are fulfilled. The former aspect necessitates a preliminary economic analysis to define the so-called “breaking point” in the balance between resources, expenditures and revenues (14). An interesting study published few years ago and including data from 20 different clinical laboratories established that consolidation becomes really cost-effective for laboratories under an estimated threshold of approximately 1.1–1.5 million tests per year, whereas no additional economic benefits are predictable by consolidation of facilities with volumes exceeding this limit (15). This inherently means that it is much more sustainable and safe to consolidate peripheral (spoke) laboratories with low volumes of tests per year into central (hub) facilities, whereas no clear advantages exist by extending this model to medium and large volume laboratories.
Many other important issues should be considered when developing a functional network of clinical laboratories (Table 1). These basically include the preliminary identification of social or political hostilities that may antagonize the changes, the development of a structured transition plan in which the laboratory staff and all putative stakeholders (i.e., policymakers, hospital administrators, medical directors, clinicians, general practitioners, labor unions, patients, diagnostic companies) must be deeply involved, the identification of performance indicators to monitor the effectiveness of the changes, along with a so-called “escape plan” in the unfortunate circumstance that something may go wrong at the end of the process. The model should also be developed taking into consideration practical aspects for ensuring that the degree of quality remains virtually unaltered (16). These basically entail a close relationship between hospital, clinics, population and laboratory, and the impact of sample transportation from one center to the other. Thanks to recent technological advances, many opportunities have recently emerged for optimizing the transportation of biological specimens, which may allow faster delivery (e.g., pneumatic tube systems, drones) (17) and continuous monitoring of sample quality by means of data loggers (18). Last but not least, the availability (or implementation) of an efficient informatics support is mandatory to allow a timely and efficient interchange of data between the various network laboratories.
Table 1
| Analysis of the local situation (costs, resources, environment) |
| Implementation of transportation system for safeguarding and monitoring sample quality |
| Availability of an efficient informatics support |
| Identification of social or political hostilities |
| Development of a structured transition plan |
| Involvement of laboratory staff and putative stakeholders |
| Identification of performance |
| Definition of an “escape plan” |
Impact of new technologies and tests
What is now clear to everybody, is that personalized medicine is not possible, nor feasible, without the support of in vitro diagnostic testing (19,20). Thanks to a greater understanding of complex biological pathways combined with remarkable technical advances, many innovative sciences have recently emerged. These basically include genomics, epigenomics, proteomics, metabolomics, theranostics (21,22). The main aspect that characterizes these types of testing is the need for dedicated, high-throughput instrumentation, coupled with information technology that would make it possible to analyze thousands or even millions of individual data at the same time, and help establishing synergies and interactions between these data. The increasing availability of these technologies would require a cultural sea change in the organization of clinical laboratories. Automation of genomics, epigenomics and proteomics is still in embryo, thus meaning that costs and demand for dedicated instrumentation and skilled personnel should be regarded as major drawbacks. Yet, many “omics” approaches, such as whole genome sequencing, still present areas of uncertainty, since the identification of some genetic polymorphisms of unknown significance may not allow to define an accurate management strategy, provided that the interplay with epigenetics and environmental influences is clearly acknowledged (22).
Conclusions
The incremental raise of health care spending is seriously threatening the sustainability of almost each public service, from education, to public health, to infrastructure. Although it is now clear and undeniable that precision (personalized) medicine will be the core opportunity for effective care in the anticipatable future, many political, economic and cultural challenges need to be overwhelmed. The route towards personalized laboratory testing will be influenced by many factors such as healthcare reforms, cost containment strategies, consolidation of laboratories and in vitro diagnostic testing, as well as the impact of new technologies and tests on the existing laboratory organization. Despite we would all agree that there is no magic bullet for succeeding in this long and winding road, system wide redesign and major synergy between laboratory professionals, diagnostic companies, patients’ associations and clinicians will be unavoidable for reaffirming the importance of personalized medicine to policymakers and hospital administrators. The message that we should jointly deliver, is that precision medicine will definitely contribute to enhancing the quality of care, but this target cannot be achieved with low resources. Healthcare systems around the globe should be embarked on a landmark effort for spending more now, for more savings later.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2016.12.01). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Plebani M. Laboratory medicine does matter in science (and medicine)… yet many seem to ignore it. Clin Chem Lab Med 2015;53:1655-6. [Crossref] [PubMed]
- Hu ZD. Laboratory medicine and precision medicine. J Lab Precis Med 2016;1:2.
- Fuchs VR. The gross domestic product and health care spending. N Engl J Med 2013;369:107-9. [Crossref] [PubMed]
- The World Bank. Health expenditure. Available online: http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS, last access, 2 December 2016.
- The World Bank. Out-of-pocket health expenditure. Available online: http://data.worldbank.org/indicator/SH.XPD.OOPC.TO.ZS, last access, 2 December 2016.
- Lippi G, Mattiuzzi C, Cervellin G. No correlation between health care expenditure and mortality in the European Union. Eur J Intern Med 2016;32:e13-4. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. Testing volume is not synonymous of cost, value and efficacy in laboratory diagnostics. Clin Chem Lab Med 2013;51:243-5. [Crossref] [PubMed]
- Rohr UP, Binder C, Dieterle T, et al. The Value of In Vitro Diagnostic Testing in Medical Practice: A Status Report. PLoS One 2016;11:e0149856 [Crossref] [PubMed]
- Lippi G, Plebani M. The "Obamanomics": a revolution in laboratory diagnostics. Clin Chem Lab Med 2010;48:741-3. [Crossref] [PubMed]
- Oberlander J. The End of Obamacare. N Engl J Med 2016; In press. [Crossref]
- Heisey R, Carroll JC. Identification and management of women with a family history of breast cancer: Practical guide for clinicians. Can Fam Physician 2016;62:799-803. [PubMed]
- Lippi G, Plebani M, Favaloro EJ. The changing face of hemostasis testing in modern laboratories: consolidation, automation, and beyond. Semin Thromb Hemost 2015;41:294-9. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. The biomarker paradigm: between diagnostic efficiency and clinical efficacy. Pol Arch Med Wewn 2015;125:282-8. [PubMed]
- Plebani M. Clinical laboratories: production industry or medical services? Clin Chem Lab Med 2015;53:995-1004. [Crossref] [PubMed]
- Barletta G, Zaninotto M, Faggian D, et al. Shop for quality or quantity? Volumes and costs in clinical laboratories. Clin Chem Lab Med 2013;51:295-301. [Crossref] [PubMed]
- Lippi G, Simundic AM. Laboratory networking and sample quality: a still relevant issue for patient safety. Clin Chem Lab Med 2012;50:1703-5. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. Biological samples transportation by drones: ready for prime time? Ann Transl Med 2016;4:92. [Crossref] [PubMed]
- Zaninotto M, Tasinato A, Padoan A, et al. Effects of sample transportation on commonly requested laboratory tests. Clin Chem Lab Med 2012;50:1755-60. [Crossref] [PubMed]
- Nice EC. From proteomics to personalized medicine: the road ahead. Expert Rev Proteomics 2016;13:341-3. [Crossref] [PubMed]
- Lippi G, Montagnana M. Laboratory and precision medicine: the reasons for the birth of a new journal. J Lab Precis Med 2016;1:3.
- Lippi G. Wisdom of theragnostics, other changes. MLO Med Lab Obs 2008;40:6. [PubMed]
- Diamandis EP, Li M. The side effects of translational omics: overtesting, overdiagnosis, overtreatment. Clin Chem Lab Med 2016;54:389-96. [Crossref] [PubMed]
Cite this article as: Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7.


