PAFIYAMA syndrome: prevention is better than cure
Intense debate has emerged about the possible negative effects of strenuous physical exercise practiced over a long period of time on fibrosis and cardiac remodeling, as well as its possible relation with the onset of certain types of arrhythmias such as atrial fibrillation (AF) (1-4). Due to its peculiar pathogenesis and accumulating data in the literature, we have recently purposed the definition of a new syndrome: strenuous endurance exercise-related AF under the acronym of ‘PAFIYAMA’ (‘paroxysmal AF in young and middle-aged athletes’). Briefly, we consider that this condition can be reached by fulfilling a number of putative (major and minor) diagnostic criteria, once common risk factors for AF and other underlying causes have been ruled out (5). The PAFIYAMA syndrome may be the consequence, at least in part, of atrial fibrotic remodeling [increased left atrial (LA) size] (6-12), among others. In addition, the clinical management should also differ from other forms of AF such as chronic AF in the aged population (5). Although the potential treatment of PAFIYAMA syndrome has deeply discussed (13), prevention is still in embryo. Unfortunately, no effective strategies are currently available for predicting cardiac maladaptations, especially of the atria (atrial dilatation and fibrosis), in those athletes who frequently practice high-intensity long-term endurance exercise. Actually, we believe that this would be the only realistic strategy to predict and prevent PAFIYAMA syndrome, or even for an early diagnosis.
In that sense, the prolongation of the PQ interval has been previously reported to be associated with exercise-induced AF (14,15), but its predictive value is poor, mainly because it depends of individual susceptibility. A wide range of novel circulating biomarkers of AF risk has been described to be also increased in endurance athletes. Former athletes also display increased values of cardiac fibrosis biomarkers such as the inhibitor of matrix metalloproteinase type I (TIMP1) (16). Galectin-3 (Gal-3) might be also involved in AF-induced atrial structural and electrical remodeling, contributing thus to AF (17,18). In fact, elevated serum values of Gal-3 have been associated with new onset AF (19) and have also been recently proposed as useful indicators for predicting AF (20).
Recent research has highlighted the pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α) as an important key in the pathophysiology of the inflammation and fibrosis observed in AF associated with high-intensity endurance exercise (21). Other circulating biomarkers such as N-terminal pro-B-type natriuretic peptide (NT-pro-BNP), fibroblast growth factor 23 (FGF-23), growth differentiation factor 15 (GDF-15), fatty acid-binding protein 4 (FABP4), interleukin-6 (IL-6), and adrenomedullin (AM) have been also associated with the incidence of AF (22). Increased plasma levels of the biomarker chitinase-3-like-1 (CHI3L1), human cartilage glycoprotein-39 (YKL-40) have been associated with more extensive LA fibrosis in patients with lone AF as well as increased risk of AF (23,24). Other inflammatory biomarkers such as C-reactive protein (CRP) and neutrophil-lymphocyte ratio (NLR) have been also purposed as predictors of AF (25).
Epigenetic is an additional area with promising perspectives. MicroRNAs (miRNAs) are a series of small noncoding RNA molecules, which regulate (either activating or inhibiting) a myriad of cell processes and body functions. miRNAs have rapidly taken the center of interest by their potential in clinical applications due to their involvement in a wide array of cardiac pathways under both physiological and pathological conditions (26), e.g., they help reconstructing ion channels by regulating gene expression in cardiomyocytes during the process of arrhythmia (27). Therefore, these miRNAs are potential biomarkers of PAFIYAMA that could enhance our understanding of its pathophysiology. For instance, circulating profibrotic miRNAs such as miRNA-21 is up-regulated by acute exercise (28). Notably, miRNA-26b is down-regulated in the right atrial tissue of AF patients thus causing pro-fibrillatory inward-rectifier potassium current up-regulation and shortening of action potential (29). Furthermore, circulating miRNA-29b is down-regulated in patients with heart failure and AF (30), which was also recently found to be reduced in the blood of healthy trained runners after completing a marathon (31). The same team of authors found that miRNA-1 and miRNA-133a, two cardiac tissue-specific miRNAs involved in myocardial ischemia-induced arrhythmia, were associated with LA diameter in trained marathon runners (32). Both markers were up-regulated and significantly correlated with LA diameter 24 hours after a marathon in well-trained athletes but not in runners with a lower training background. It was hence suggested that the level of miRNA expression may explain, at least partly, the discrepancy between ‘beneficial moderate exercise’ and ‘potentially harmful’ strenuous endurance, and that circulating miRNAs could serve as biomarkers of pro-arrhythmogenic signaling leading to atrial enlargement after long-term strenuous endurance exercise. The expression of miRNA-222 is also triggered by physiological hypertrophy (33), and differs between endurance and strength athletes, since it is overexpressed in endurance athletes and reduced in strength athletes (34).
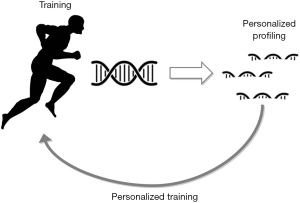
The so-called ‘resistance exercisers’ (recreational or amateur), an increasing population group following long-duration and high-intensity resistance training, probably require more cardiological studies and monitoring to the best of our knowledge. Certain number of exercisers are more vulnerable to potentially detrimental cardiac maladaptations, i.e., LA dilation, and hence are exposed to greater risks from a cardiovascular perspective, which mostly entails certain types of arrhythmias such as AF. Certainly, there are individuals who adapt better than others with the same training loads due to genetic, epigenetic, environmental and adaptive factors. It is hence crucial to identifying a safety training zone and establishing the individual threshold according to their individual susceptibility as well as the recommended recovery periods. Likewise, the training characteristics could be individually adapted in order to lead to a healthy life. Unfortunately, current imaging techniques do not allow us to reliably anticipate this process of maladaptation and pathologic atrial fibrosis and/or remodeling. However, some circulating molecules including miRNAs (see Table 1 for a summary) are biomarkers which can be measured in blood and, based on previous observations, their assessment may help determine which subjects are at greater risk or are undergoing a maladaptive cardiac process in response to the type, intensity and duration of the exercise performed. Furthermore, the miRNAs regulate the traduction process from the messenger RNAs to proteins at cellular level that may be involved in this particular process. It would hence be possible to increase or decrease the expression of these miRNAs by specific interventions such as appropriate training programs and, therefore, modulate this process in an individualized manner according to the individual risk (Figure 1). Based on these observations and from our viewpoint, it would be very useful to identify feasible biomarkers which can be used for monitoring and predicting cardiac maladaptation to training, particularly of the atria, so preventing PAFIYAMA syndrome. Additionally, provided that future studies will confirm these assumptions, and one of these is already ongoing in our institution (Run For Science, http://www.dsnm.univr.it/?ent=iniziativa&id=5382&lang=en), personal training based on certain biomarkers and epigenetics variations may even be recommended as a part of the athlete’s annual evaluation along with stress test, electrocardiography and echocardiography.
Table 1
| Molecules |
| Tissue metalloproteinase inhibitor 1 or inhibitor of matrix metalloproteinase type I (TIMP1) |
| Galectin-3 (Gal-3) |
| N-terminal pro-B-type natriuretic peptide (NT-proBNP) |
| fibroblast growth factor 23 (FGF-23) |
| Growth differentiation factor 15 (GDF-15) |
| Fatty acid-binding protein 4 (FABP4) |
| Interleukin-6 (IL-6) |
| Adrenomedullin (AM) |
| chitinase-3-like-1 (CHI3L1), human cartilage glycoprotein-39 (YKL-40) |
| C-reactive protein (CRP) |
| Neutrophil-lymphocyte ratio (NLR) |
| miRNAs |
| miRNA-21 |
| miRNA-26 |
| miRNA-29b |
| miRNA-1 |
| miRNA-133a |
| miRNA-222 |
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2016.12.02). Giuseppe Lippi serves as Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Fabian Sanchis-Gomar serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from December 2016 to November 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanchis-Gomar F, Pareja-Galeano H, Santos-Lozano A, et al. Strenuous exercise and the heart: are we not seeing the wood for the trees? Int J Cardiol 2014;176:1304-5. [Crossref] [PubMed]
- Sanchis-Gomar F, Joyner MJ, Lucia A. Letter by Sanchis-Gomar et al Regarding Article, "Cardiac Remodeling in Response to 1 Year of Intensive Endurance Training Circulation 2015;132:e146 [Crossref] [PubMed]
- Sanchis-Gomar F, Pareja-Galeano H, Santos-Lozano A, et al. Strenuous Exercise Worse Than Sedentarism? J Am Coll Cardiol 2015;65:2673-4. [Crossref] [PubMed]
- Sanchis-Gomar F, Pareja-Galeano H, Santos-Lozano A, et al. Long-term Strenuous Endurance Exercise and the Right Ventricle: Is It a Real Matter of Concern? Can J Cardiol 2015;31:1304.e1 [Crossref] [PubMed]
- Sanchis-Gomar F, Perez-Quilis C, Lippi G, et al. Atrial fibrillation in highly trained endurance athletes - Description of a syndrome. Int J Cardiol 2017;226:11-20. [Crossref] [PubMed]
- Sanchis-Gomar F, López-Ramón M, Alis R, et al. No evidence of adverse cardiac remodeling in former elite endurance athletes. Int J Cardiol 2016;222:171-7. [Crossref] [PubMed]
- Sanchis-Gomar F, Garatachea N, Catalán P, et al. LA Size in Former Elite Athletes. JACC Cardiovasc Imaging 2016;9:630-2. [Crossref] [PubMed]
- Iskandar A, Mujtaba MT, Thompson PD. Left Atrium Size in Elite Athletes. JACC Cardiovasc Imaging 2015;8:753-62. [Crossref] [PubMed]
- Elliott AD, Mahajan R, Lau DH, et al. Atrial Fibrillation in Endurance Athletes: From Mechanism to Management. Cardiol Clin 2016;34:567-78. [Crossref] [PubMed]
- D'Andrea A, Bossone E, Radmilovic J, et al. Exercise-Induced Atrial Remodeling: The Forgotten Chamber. Cardiol Clin 2016;34:557-65. [Crossref] [PubMed]
- Sanchis-Gomar F, Lucia A. Pathophysiology of atrial fibrillation in endurance athletes: an overview of recent findings. CMAJ 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Patel DA, Lavie CJ, Milani RV, et al. Clinical implications of left atrial enlargement: a review. Ochsner J 2009;9:191-6. [PubMed]
- Guasch E, Mont L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nat Rev Cardiol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Grimsmo J, Grundvold I, Maehlum S, et al. High prevalence of atrial fibrillation in long-term endurance cross-country skiers: echocardiographic findings and possible predictors--a 28-30 years follow-up study. Eur J Cardiovasc Prev Rehabil 2010;17:100-5. [Crossref] [PubMed]
- Van Buuren F, Mellwig KP, Faber L, et al. The occurrence of atrial fibrillation in former top-level handball players above the age of 50. Acta Cardiol 2012;67:213-20. [PubMed]
- Lindsay MM, Dunn FG. Biochemical evidence of myocardial fibrosis in veteran endurance athletes. Br J Sports Med 2007;41:447-52. [Crossref] [PubMed]
- Ho JE, Yin X, Levy D, et al. Galectin 3 and incident atrial fibrillation in the community. Am Heart J 2014;167:729-34.e1. [Crossref] [PubMed]
- Takemoto Y, Ramirez RJ, Yokokawa M, et al. Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes. JACC Basic Transl Sci 2016;1:143-54. [Crossref] [PubMed]
- Chen D, Procter N, Goh V, et al. New onset atrial fibrillation is associated with elevated galectin-3 levels. Int J Cardiol 2016;223:48-9. [Crossref] [PubMed]
- Hijazi Z, Oldgren J, Siegbahn A, et al. Application of Biomarkers for Risk Stratification in Patients with Atrial Fibrillation. Clin Chem 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat Commun 2015;6:6018. [Crossref] [PubMed]
- Lind L, Sundström J, Stenemo M, et al. Discovery of new biomarkers for atrial fibrillation using a custom-made proteomics chip. Heart 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Marott SC, Benn M, Johansen JS, et al. YKL-40 levels and atrial fibrillation in the general population. Int J Cardiol 2013;167:1354-9. [Crossref] [PubMed]
- Canpolat U, Aytemir K, Hazirolan T, et al. Serum YKL-40 as a Marker of Left Atrial Fibrosis Assessed by Delayed Enhancement MRI in Lone Atrial Fibrillation. Pacing Clin Electrophysiol 2015;38:1386-95. [Crossref] [PubMed]
- Paquissi FC. The Predictive Role of Inflammatory Biomarkers in Atrial Fibrillation as Seen through Neutrophil-Lymphocyte Ratio Mirror. J Biomark 2016;2016:8160393
- Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol 2009;6:419-29. [Crossref] [PubMed]
- van Rooij E, Sutherland LB, Qi X, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007;316:575-9. [Crossref] [PubMed]
- Baggish AL, Hale A, Weiner RB, et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 2011;589:3983-94. [Crossref] [PubMed]
- Luo X, Pan Z, Shan H, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest 2013;123:1939-51. [Crossref] [PubMed]
- Dawson K, Wakili R, Ordög B, et al. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 2013;127:1466-75, 1475e1-28.
- Clauss S, Wakili R, Hildebrand B, et al. MicroRNAs as Biomarkers for Acute Atrial Remodeling in Marathon Runners (The miRathon Study--A Sub-Study of the Munich Marathon Study). PLoS One 2016;11:e0148599 [Crossref] [PubMed]
- Carpenter A, Frontera A, Bond R, et al. Vagal atrial fibrillation: What is it and should we treat it? Int J Cardiol 2015;201:415-21. [Crossref] [PubMed]
- Liu X, Xiao J, Zhu H, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 2015;21:584-95. [Crossref] [PubMed]
- Wardle SL, Bailey ME, Kilikevicius A, et al. Plasma microRNA levels differ between endurance and strength athletes. PLoS One 2015;10:e0122107 [Crossref] [PubMed]
Cite this article as: Perez-Quilis C, Lippi G, Mena S, Löllgen H, García-Giménez JL, Sanchis-Gomar F. PAFIYAMA syndrome: prevention is better than cure. J Lab Precis Med 2016;1:8.