
The emerging value of molecular forms of B-type natriuretic peptide in heart failure
Brain natriuretic peptide (BNP) has an established role in diagnosing heart failure (HF) and has shown to provide independent prognostic information. However, its prognostic value has limitations, leading to the development of other prognostic biomarkers such as ST2 and galectin-3 (1,2). With recent advances, we have gained more understanding into the bioactivity and metabolism of BNP. In different individuals, there are actually many BNP fragments in the circulation as a response to the degradation process. From the success of angiotensin receptor neprilysin inhibitors (ARNI) in HF treatment as shown in the PARADIGM-HF trial (3), we have learned that controlling BNP metabolism is associated with better clinical outcome. However, it seems that this is just the tip of the iceberg in the complex world of BNP metabolism. In the article published by Toru Suzuki and colleagues in the Journal of Clinical Chemistry (4), it was highlighted that the molecular forms of BNP not only represent the products after degradation, but that they also play a role as prognostic biomarkers. This marks a promising and exciting a new avenue in the evaluation and treatment for patients with cardiovascular diseases.
Paradox
The body’s natural response to HF involves activation of the sympathetic nervous system (SNS), the renin-angiotensin-aldosterone system (RAAS), and the natriuretic peptide system (NPS). NPS is supposed to counteract the vasoconstrictive effects of the RAAS and SNS by stimulating vasodilatation. However, in advanced HF, the remarkably activated NPS fails to accomplish this important balance. This was previously attributed to an inadequate response to the activated NPS, but now we have gained more understanding into the mechanisms underlying this paradox.
BNP originates from PreproBNP. PreproBNP1-134 is cleaved to form a signal peptide and ProBNP1-108 (Figure 1). ProBNP1-108 is subsequently cleaved by either corin or furin into NT-proBNP1-76 and BNP1-32. However, recent studies have revealed that the commercialized immunoassay for BNP1-32 actually detects any BNPs that express the epitope identified by the immunoassay, rather than specifically measuring the concentration of bioactive BNP1-32 in the circulation (5). This would explain why despite high BNP levels, the beneficial effects on vasodilation, natriuresis etc. are not seen. In fact, the actual bioactive BNP1-32 level could very well be extremely low, however we are not equipped to discuss this issue, as it is not measured as a routine. Furthermore, not only is the real BNP1-32 level over-estimated, the degradation of BNP1-32 is also quicker than previously thought. These above circumstances would explain the so-called BNP paradox. The expansion of knowledge on BNP has been further advanced to a new era in which we should consider both the function and quantity of BNP1-32 degraded fragments, the so-called BNP molecular forms.
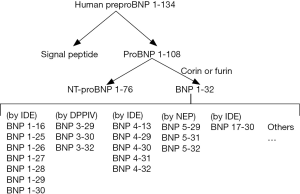
The BNP paradox involves a few critical issues: (I) what is the major form of measured BNP in the circulation? Is it individualized? (II) What is the critical step of producing bioactive BNP1-32 from the inactive BNP1-180 form? Which enzyme is responsible? Is it gene regulated? (III) Are BNP molecular forms bioactive? If so, which has the strongest function and has what effect? (IV) Are the levels manipulated by specific degrading enzymes or their corresponding inhibitors? (V) Which BNP molecular forms are involved in the ARNI effect? And (VI) will a new panel measuring BNP molecular forms be helpful? How are they to be applied in the clinical setting?
The inaccurate quantification of BNP1-32 in circulation has lead to confusion in the interpretation of BNP values in current publications. Derived from BNP1-108 cleaved by corin, BNP1-32 can be degraded to BNP3-32 by Dipeptidyl peptidase-4 (DPPIV), to BNP5-32 by neutral endopeptidase (neprilysin, NEP), to BNP8-32 by meprin (not present in humans), and to smaller degradation peptides by insulin degrading enzyme (IDE) (Figure 1) (6). To confuse matters, BNP1-32 immunoassays also detect proBNP1-108, and BNP3-32, but not NT-proBNP1-76.
In patient plasma, the concentration of proBNP1-108 is almost 6.3 times that of BNP1-32, suggesting proBNP1-108 circulates in much higher concentrations than BNP1-32, and is the major BNP form in HF patients (5,7). Other mechanisms associated with this disproportional ratio include rapid degradation of BNP1-32, impaired production of BNP1-32 from proBNP1-108 (8), and inactivated convertases seen in HF, such as corin. For example, it has been shown that expression of the corin alleles cause impairment in the processing of proBNP1-108. More clinically relevant, survival free from death or first HF-related hospitalization was significantly worse in carriers of the corin allele compared to non-carriers (9).
Which BNP is bioactive?
Not all processed and degraded forms of BNP have ability to bind to the receptor and show activity. So far, we know that the functioning bioactive BNP is BNP1-32, whereas proBNP1-108 and NT pro-BNP1-76 are not. However, the position is not well established for other BNP fragments. There has been some data on BNP1-32, BNP3-32, BNP8-32, proBNP1-108 (in decreasing order of activity). Mature BNP1-32 is most active; BNP3-32 shows reduced natriuresis, diuresis and lack vasodilating actions (10). Compared to BNP1-32, several BNP forms generated by IDE degradation have been shown to activate bioactive reactions 7-fold greater in some in vitro experiments (11). However, information is not adequate for other molecular forms such as BNP5-32 and data regarding functional capacity have not all been consistent. Jessica O’Rear et al. investigated the bioactivity of a few proteolysed BNP peptides, including BNP4-32, BNP5-32, BNP5-31, BNP1-25, and BNP1-26, and found no differences compared with BNP1-32 (unpublished data).
In the era of ARNI
In the PARADIGM-HF trial, when compared with enalapril, sacubitril (a neprilysin inhibitor)/valsartan treatment resulted in decreased mortality and hospitalizations for HF along with sustained reductions in circulating troponin-T levels (3). These findings have recently drawn great attention to the role of ARNI. In the era of ARNI, BNP levels are significantly increased with blockage of its degradation. However, the true bioactive BNP level is not well explored. The neprilysin inhibitor component in ARNI is supposed to decrease the degradation of BNP1-32 to BNP5-32, thus leading to increased levels of BNP1-32. However without measuring the different BNP fragments in patients on ARNI, we won’t know the actual events. Studies elucidating the changes in concentration of these BNP fragments will shed light on how they exert their beneficial effects in HF patients. This will be important since not all patients have the same response to ARNI.
Better prognostic value of BNP fragments
Although the methodology of quantitating BNP molecular forms based on mass spectrometry is established, it is not commercialized and popular at the present time. Accordingly, their clinical value is not yet well explored. The article published by Toru Suzuki demonstrated the prognostic value of BNP5-32 in HF patients, comparable to NT-proBNP. Furthermore, the low interference of BNP5-32 with kidney function may contribute to the superior prognostic value of BNP fragments than the traditional NT-proBNP (4). This really opens an avenue of a better outcome assessment based on quantifying the molecular forms of BNP1-32. Siriwardena et al. reported the potential of using BNP signal peptide as a biomarker of myocardial ischemia (12). Others have demonstrated that some BNP fragments are valuable in identifying restenosis post-coronary intervention (13). These fragments exhibit a very promising role in various situations and their applications should be further explored and verified.
Clinical applications
It seems reasonable to set up a commercialized panel to measure BNP fragments. With this approach, we can definitely explore the multifaceted details of using BNP fragments for clinical purposes beyond identifying coronary artery restenosis, myocardial ischemia/infarction and assessing prognosis. It is plausible that the utilization of this panel can allow us to better understand the mechanisms underlying the interaction of multiple drug effects, or even develop better pharmaceutical interventions via manipulation of blood BNP fragment levels. The use of mass spectrometry as the primary quantification facility actually broadens how we view the complexity of treating patients with HF. For example, metabolomics is an emerging science in providing big data information on metabolism in HF; studies have shown the promising diagnostic and prognostic value of metabolomics in HF (14). It is likely that a systemic approach based on a platform integrating all these molecules will be the way forward, providing informative assessments of different HF situations and determining response to a variety of medications.
Acknowledgments
Funding: This study was supported in part by the Ministry of Science and Technology, R.O.C. (MOST 105-2314-B-182-046-MY2) and Chang Gung Memorial Hospital (CMRPG2C0312, 2C0351, 2C0361, CMRPD1A0522, CMRPG2F0081 and CMRPG2A0172).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Executive Editor Zhi-De Hu (Department of Laboratory Medicine, General Hospital of Ji’nan Military Region, Ji’nan, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.05.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rubattu S, Triposkiadis F. Resetting the neurohormonal balance in heart failure (HF): the relevance of the natriuretic peptide (NP) system to the clinical management of patients with HF. Heart Fail Rev 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Wang CH, Yang NI, Liu MH, et al. Estimating systemic fibrosis by combining galectin-3 and ST2 provides powerful risk stratification value for patients after acute decompensated heart failure. Cardiol J 2016; [Epub ahead of print]. [PubMed]
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004. [Crossref] [PubMed]
- Suzuki T, Israr MZ, Heaney LM, et al. Prognostic Role of Molecular Forms of B-Type Natriuretic Peptide in Acute Heart Failure. Clin Chem 2017;63:880-6. [Crossref] [PubMed]
- Niederkofler EE, Kiernan UA, O’Rear J, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail 2008;1:258-64. [Crossref] [PubMed]
- Ichiki T, Huntley BK, Burnett JC Jr. BNP molecular forms and processing by the cardiac serine protease corin. Adv Clin Chem 2013;61:1-31. [Crossref] [PubMed]
- Seferian KR, Tamm NN, Semenov AG, et al. The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clin Chem 2007;53:866-73. [Crossref] [PubMed]
- Rame JE, Drazner MH, Post W, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension 2007;49:857-64. [Crossref] [PubMed]
- Rame JE, Tam SW, McNamara D, et al. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail 2009;2:541-8. [Crossref] [PubMed]
- Boerrigter G, Costello-Boerrigter LC, Harty GJ, et al. B-type natriuretic peptide 8-32, which is produced from mature BNP 1-32 by the metalloprotease meprin A, has reduced bioactivity. Am J Physiol Regul Integr Comp Physiol 2009;296:R1744-50. [Crossref] [PubMed]
- Ralat LA, Guo Q, Ren M, et al. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. J Biol Chem 2011;286:4670-9. [Crossref] [PubMed]
- Siriwardena M, Kleffmann T, Ruygrok P, et al. B-type natriuretic peptide signal peptide circulates in human blood: evaluation as a potential biomarker of cardiac ischemia. Circulation 2010;122:255-64. [Crossref] [PubMed]
- Fujimoto H, Suzuki T, Aizawa K, et al. Processed B-type natriuretic peptide is a biomarker of postinterventional restenosis in ischemic heart disease. Clin Chem 2013;59:1330-7. [Crossref] [PubMed]
- Cheng ML, Wang CH, Shiao MS, et al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol 2015;65:1509-20. [Crossref] [PubMed]
Cite this article as: Yang NI, Wang CH. The emerging value of molecular forms of B-type natriuretic peptide in heart failure. J Lab Precis Med 2017;2:23.


