
Anti-carbamylated protein antibodies in rheumatoid arthritis patients are reactive to specific epitopes of the human fibrinogen β-chain
In this issue of Arthritis & Rheumatology, Jones et al. aim to identify the presence of immunodominant epitope(s) within human fibrinogen in regards to the anti-carbamylated protein antibodies (ACarPA) reactivity in rheumatoid arthritis (RA) patient sera.
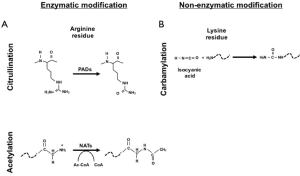
RA is a chronic inflammatory autoimmune disease. Beside synovial inflammation one of the hallmarks of RA is the presence of autoantibodies. Rheumatoid factor (RF), which is an autoantibody against the Fc portion of immunoglobulins, was the first to be extensively characterized and to be included in the 1987 American College of Rheumatology (ACR) classification criteria for RA (1-3). Many autoantibodies in RA are directed against post-translational modification (PTM) such as citrullination, carbamylation and acetylation. PTM can be the result of enzymatic modification (e.g., citrullination of arginine by peptidylarginine deiminase enzyme) (Figure 1A) or non-enzymatic addition (e.g., carbamylation of lysine to homocitrulline through isocyanic acid) (Figure 1B) (4,5).
Autoantibodies directed against citrullinated antigens, known as anti-citrullinated protein antibodies (ACPA), are strongly associated with the risk of RA. Their presence can be detected years before the disease clinical manifestation and is a good diagnostic marker for RA (5,6). Nowadays, the ACR EULAR 2010 classification criteria include both RF and ACPA (6). However, ACPA are directed against a wide range of citrullinated antigens such as fibrinogen, vimentin, α-enolase, filaggrin and histones, and are polyreactive towards citrulline residues (7-9). Moreover, it is still not clear which is the specific citrullinated antigen that triggers autoimmunity and drives the disease in vivo. Therefore, the reactivity characterization of autoantibodies towards other PTM has risen interest.
To date, ACarPA are the best studied autoantibodies in RA after ACPA. These autoantibodies can be detected in around 45% of early arthritis patients and they can be found in both ACPA positive and ACPA negative patients (5,10-12). They can be cross-reactive with ACPA which has made difficult their immunoreactivity characterization (7). However, it is now accepted that ACarPA and ACPA represent two distinct classes of autoantibodies in RA (5,10).
As mentioned above, carbamylation is a non-enzymatic PTM mediated by the binding of isocyanic acid to the –NH2 group of the side chain of the lysine which brings to the formation of homocitrulline (Figure 1B) (5,13). Isocyanic acid can form from thiocyanate via myeloperoxidase which is well known to increase during inflammation (5,14). Like ACPA, ACarPA can be detected before the clinical onset of the disease and be a predictor of radiographic progression (7,15,16). ACarPA were first characterised in 2011 using as antigen the fetal calf serum (FCS) (5,15). Afterwards, human carbamylated antigens were used including fibrinogen, filaggrin, vimentin, enolase, and collagen (5).
As shown in the work of Jones et al. (7,17), ACarPA seem to be primarily directed against human fibrinogen. This protein is formed by two copies of three distinctive chains (α, β, and γ) and it is a well characterised autoantigen in RA recognized also by ACPA. In this article, the authors show that some ACarPA can be specifically directed towards homocitrullinated peptides in the β-chain of fibrinogen, thus supporting the presence of immunodominant epitopes. Using serum from RA patients ACPA and RF negative, the authors have shown that ACarPA positive sera react towards different specific peptides containing homocitrullines in the fibrinogen β-chain and not towards the fibrinogen α and γ chain. Interestingly, selected sera were not polyreactive with homocitrullines between peptides confirming the presence in some patients of dominant epitopes within the β-chain of fibrinogen. Using competition ELISA assays, the authors found that selected carbamylated peptides within fibrinogen were able to compete with carbamylated FCS in the binding to ACarPA. In particular, the fibrinogen peptides K77AAATQKKVER87 and A76KAAATQKKVERKAP90 with a single carbamylated lysine at position 83 mediated over 30% inhibition (7). Interestingly, they observed no inhibition in the absence of homocitrulline at position 83 only in a subset of RA patients (35%), thus suggesting the presence of a dominant epitope at this position (7). In addition to the carbamylated lysine 83, homocitrulline at position 367 and 374 were also identified by the authors as dominant epitopes but only in serum samples reacting with carbamylated lysine at position 83 (7).
All together, these results could have significant utility since a better characterization of the autoantibody reactivity in RA might be useful to identify different groups of RA patients which share the same autoantibody profile, thus clinical disease course and maybe disease outcome. In particular, it could address better the role of ACarPA in the pathogenesis of RA as well as be used as an additional diagnostic marker.
As stated by the authors (7), it is still unclear whether: (I) ACarPA are a subset of cross-reactive ACPA in the pre-clinical phase of the disease; (II) the cross-reactivity with ACPA might be associated with a better or worst radiographic outcome; (III) ACarPA change with the disease activity; and (IV) there is a real specificity towards fibrinogen or ACarPA can react with some other antigens. Clearly, more studies on anti-carbamylated autoantibodies need to be performed in order to have a better understanding of the ACarPA immunoreactivity and also function i.e., it is still not known whether ACarPA play a pathogenic role in RA.
In conclusion, the authors of this issue of Arthritis & Rheumatology have shown that ACarPA can react towards specific carbamylated β-chain fibrinogen peptides, thus supporting a certain level of ACarPA specificity in RA that will be important to validate with further experiments.
Acknowledgments
Funding: None.
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Min Yang (Department of Laboratory Medicine, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China).
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Min Yang (Department of Laboratory Medicine, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.06.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Edelman GM, Kunkel HG, Franklin EC. Interaction of the rheumatoid factor with antigen-antibody complexes and aggregated gamma globulin. J Exp Med 1958;108:105-20. [Crossref] [PubMed]
- Vaughan JH. Behavior of the rheumatoid arthritis agglutinating factor with immune precipitates. J Immunol 1956;77:181-8. [PubMed]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. [Crossref] [PubMed]
- Shi J, van Veelen PA, Mahler M, et al. Carbamylation and antibodies against carbamylated proteins in autoimmunity and other pathologies. Autoimmun Rev 2014;13:225-30. [Crossref] [PubMed]
- Trouw LA, Rispens T, Toes RE. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nat Rev Rheumatol 2017;13:331-9. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81. [Crossref] [PubMed]
- Jones JD, Hamilton BJ, Rigby WF. Anti-carbamylated protein antibodies in rheumatoid arthritis patients are reactive to specific epitopes of the human fibrinogen beta-chain. Arthritis Rheumatol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One 2012;7:e35296 [Crossref] [PubMed]
- Corsiero E, Bombardieri M, Carlotti E, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis 2016;75:1866-75. [Crossref] [PubMed]
- Shi J, Willemze A, Janssen GM, et al. Recognition of citrullinated and carbamylated proteins by human antibodies: specificity, cross-reactivity and the 'AMC-Senshu' method. Ann Rheum Dis 2013;72:148-50. [Crossref] [PubMed]
- Reed E, Jiang X, Kharlamova N, et al. Antibodies to carbamylated alpha-enolase epitopes in rheumatoid arthritis also bind citrullinated epitopes and are largely indistinct from anti-citrullinated protein antibodies. Arthritis Res Ther 2016;18:96. [Crossref] [PubMed]
- Verheul MK, Shiozawa K, Levarht EW, et al. Anti-carbamylated protein antibodies in rheumatoid arthritis patients of Asian descent. Rheumatology (Oxford) 2015;54:1930-2. [Crossref] [PubMed]
- Jaisson S, Pietrement C, Gillery P. Carbamylation-derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin Chem 2011;57:1499-505. [Crossref] [PubMed]
- Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 2007;13:1176-84. [Crossref] [PubMed]
- Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A 2011;108:17372-7. [Crossref] [PubMed]
- Brink M, Verheul MK, Ronnelid J, et al. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther 2015;17:25. [Crossref] [PubMed]
- Scinocca M, Bell DA, Racape M, et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol 2014;41:270-9. [Crossref] [PubMed]
Cite this article as: Corsiero E, Bombardieri M, Pitzalis C. Anti-carbamylated protein antibodies in rheumatoid arthritis patients are reactive to specific epitopes of the human fibrinogen β-chain. J Lab Precis Med 2017;2:38.


