
Discrepant impacts of age and gender factors on serum pepsinogens and gastrin-17 levels
Introduction
Pepsinogen (PG) is a family of digestive enzyme precursors secreted by gastric mucosa that contains two subgroups, pepsinogen I (PGI) and pepsinogen II (PGII) (1). Both PGI and PGII are secreted by cervical mucosal cells and chief cells. PGII is also secreted by mucus cells of the gastric antrum and Brunner gland of the proximal duodenum (2). The serum concentration of PGs directly reflects their secretion levels and is an indicator of gastric mucosal damage such as atrophic gastritis (3). Gastrin-17 (G-17) is a gastrointestinal hormone secreted by G cells mainly in the gastric antrum and it primarily stimulates gastric acid production as well as secretion of PGs (4).
A combined test for serum PGI, PGII, and G-17 is now helpful for diagnosis, early prediction or treatment monitoring of gastric-related diseases including gastric cancer, and contribute to the healthy management of the residents (5). However, factors that may influence the interpretation of these test results have not been fully discussed to date. In this study, we collected serum PGI, PGII, and G-17 results from 2,732 residents of Nanjing, China, to evaluate factors that may correlate with these values.
Methods
Clinical sample treatment
This was a retrospective study that included 2,732 healthy residents without diagnosed gastric disease or other apparent diseases and who received a yearly health check-up. The study was approved by Ethics Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University. The study population consisted of 1,348 males (median age: 63 years) and 1,384 females (median age: 61 years) and was stratified by age into three groups: ˂45, 45–64, and ≥65 years. Peripheral blood samples were collected in the morning and were centrifuged at 3,000 rpm for 5 minutes to isolate the serum. Samples were stored at −20 °C until analysis.
Enzyme-linked immunosorbent assay (ELISA)
The serum PGI, PGII, and G-17 detection kits were from Biohit Corporation (Helsinki, Finland). We used the ELISA kits according to the manufacturer’s instructions. Reference ranges for PGI, PGII, G-17, and PGR were 70–165 µg/L, 3–15 µg/L, 1–15 pmol/L, and 7–20 respectively. The functional sensitivities claimed by the manufacture are 92% for PGI, 95% for PGII, and 95% for G-17 respectively.
Statistical analysis
We used SPSS 17.0 software for data analysis. All data were analyzed by Kolmogorov-Smirnov for normality test. One-way analysis of variance (ANOVA) and Student’s t-test was applied for comparison between groups with preliminary results transferred by the Ln method. Spearman correlation was used to determine relation between age and parameters. Single factor analysis of variance and Tukey test were used for subgroups comparison. A P value less than 0.05 was considered statistically significant. We used non-parametric 95% percentile method to calculate reference intervals.
Results
Patient characteristics
Among 2,732 serum samples analysed, the abnormality rates of PGI, PGII, G-17, and PGR were assessed by age and gender respectively (Table 1). The prevalence of PGI, PGII, and G-17 was 16.44% in the ˂45 years group, 29.03% in the 45–64 years group, and 36.24% in the ≥65 years group. Meanwhile, the total abnormality rates were 39.79% in males and 41.87% in females respectively.
Table 1
| Variables | Abnormal PGI | Abnormal PGII | Abnormal G-17 | Abnormal PGR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| Age (years) | |||||||||||
| <45 (n=537) | 177 | 6.48 | 104 | 3.81 | 168 | 6.15 | 97 | 3.55 | |||
| 45–64 (n=907) | 309 | 11.31 | 206 | 7.54 | 278 | 10.18 | 203 | 7.43 | |||
| ≥65 (n=1,288) | 418 | 15.30 | 370 | 13.54 | 202 | 7.39 | 452 | 16.54 | |||
| Gender | |||||||||||
| Male (n=1,348) | 414 | 15.15 | 360 | 13.18 | 313 | 11.46 | 371 | 13.58 | |||
| Female (n=1,384) | 490 | 17.94 | 319 | 11.68 | 335 | 12.26 | 384 | 14.06 | |||
PGI, pepsinogen I; PGII, pepsinogen II; G-17, gastrin-17.
Serum PGs, PGR, and G-17 levels across different age groups
The serum PGs, PGR, and G-17 levels were analysed simultaneously in all samples. Median PGI levels were 100.4 µg/L (˂45 years group), 115.1 µg/L (45–64 years group), and 126.2 µg/L (≥65 years group); median PGII levels were 9.4 µg/L (˂45 years group), 11.3 µg/L (45–64 years group), and 14.4 µg/L (≥65 years group); median G-17 levels were 7.1 pmol/L (˂45 years group), 9.2 pmol/L (45–64 years group), and 13.6 pmol/L (≥65 years group); and median PGRs values were 14.0 (˂45 years group), 13.2 (45–64 years group), and 11.6 (≥65 years group) (Figure 1). Serum PGs and G-17 levels were highest in the ≥65 years group while PGR decreased with age.

Age effect on the parameters was confirmed by Spearman correlation analysis (Table 2). Serum PGI, PGII, and G-17 values showed slightly positive correlation with age, while PGR negatively correlated. When gender factor was analyzed separately, we found that the correlation between parameters and age turned more significantly except Female G-17.
Table 2
| Parameters | Male (n=1,348) | Female (n=1,384) | |||
|---|---|---|---|---|---|
| r | P value | r | P value | ||
| PGI | 0.19 | <0.0001 | 0.19 | <0.0001 | |
| PGII | 0.23 | <0.0001 | 0.2 | <0.0001 | |
| PGR | −0.15 | <0.0001 | −0.11 | <0.0001 | |
| G-17 | 0.09 | <0.0005 | −0.04 | 0.08 | |
PGI, pepsinogen I; PGII, pepsinogen II; G-17, gastrin-17.
Comparison of serum PGs, PGR, and G-17 levels based on gender
As shown in Figure 2, median serum PGI and PGII levels in males were 120.4 and 11.9 µg/L, respectively, while those in females were 103.1 and 10.3 µg/L respectively. However, serum G-17 levels were significantly lower in men than in women (8.2 vs. 10.1 pmol/L). There was no significant difference in gender for PGR. Further, we combined both influence factors to re-analyze the data. As shown in Figure 3, the overall trend was identical to that of Figure 2, and significant differences only emerged in ˂45 years group and 45–64 years group.

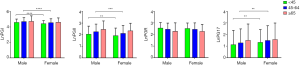
Reference intervals of PGI, PGII, G17 and PGR based on age and/or gender
Finally, we tried to establish compatible reference intervals to distinguish between people of different age and gender. We chose the minimum age 15 for a starting point and divided people every 10 years old. Comparison of each parameter between groups was accomplished by ANOVA and Turkey test. If there was no significant result, adjacent groups would be merged. The reference intervals were listed in Table 3.
Table 3
| Age (years) | 2.5th | 97.5th |
|---|---|---|
| PGI (µg/L) | ||
| Male | ||
| 15–49 | 32.2 | 261.0 |
| 50–59 | 45.3 | 299.7 |
| ≥60 | 34.9 | 315.8 |
| Female | ||
| 10–39 | 37.9 | 200.8 |
| 40–59 | 31.5 | 234.5 |
| ≥60 | 33.8 | 265.1 |
| PGII (µg/L) | ||
| Male | ||
| 15–29 | 2.8 | 38.1 |
| 30–49 | 2.3 | 35.3 |
| 50–59 | 3.1 | 40.7 |
| ≥60 | 3.4 | 44.9 |
| Female | ||
| 10–59 | 2.3 | 33.5 |
| ≥60 | 3.3 | 40.2 |
| G-17 (male) | ||
| 15–49 | 0.5 | 46.1 |
| 50–59 | 0.1 | 58.3 |
| ≥60 | 0.3 | 80.3 |
| PGR (pmol/L) | ||
| 10–29 | 2.5 | 29.4 |
| 30–59 | 3.5 | 27.7 |
| ≥60 | 3.2 | 24.1 |
PGI, pepsinogen I; PGII, pepsinogen II; G-17, gastrin-17.
Discussion
In this study, we found that serum PGs and G-17 levels gradually increased with age, which is consistent with a previous study performed by Sun et al. (6). Serum PGs are considered to be sensitive biological indicators of lesions in the gastric fundus mucosa (7). Levels of PGs are elevated in the early stages of atrophic gastritis, which is the first step of a cascade that leads to gastric cancer. As reported by Lee et al. (8), when chief cells are replaced by pyloric gland cells as inflammation progresses, PGI levels decline, but PGII levels increase, leading to a decline in PGR (consistent with our findings). Kang et al. found that serum levels of PGs are positively correlated with chronic inflammation and demonstrated that increased PGI or PGII levels with decreased PGR was correlated with the severity of inflammation (9). Gastric mucosal inflammation in the antrum and its extension to the corpus is associated with increased serum PGII but is not related to PGI (8,10). The results explained why higher levels of PGs were correlated with a higher incidence of chronic gastritis with age. Serum G-17 is a marker of atrophic gastritis especially for atrophy sites evaluation. When mucosal atrophy of the gastric corpus occurs, G cells shrink and secretion decreases. Serum G-17 level has been found to be negatively correlated with chronic inflammation of the gastric antrum. Combined detection of G-17 with PGs served as a clinical tool for gastric atrophy assessment (11). Another interesting finding in this study is that serum PGs levels of males were significantly higher than those of females. But due to follow-up breach, information such as diet, smoking status and drinking habit were incomplete, and these factors should be considered when gastric test results are interpreted. We believed that higher PGs levels in male were somehow related to unique customs of Asian countries (12), and Chinese males indeed tend to have gastric discomfort.
During this study, we noticed that females pay sufficient attention as males to annual screening for gastric disease. A set of new reference intervals, we suggested based on both age and gender factors, should be further verified clinically to guide the right access to reliable laboratory results for diagnose.
Acknowledgments
We are grateful to the technical support from National Key Clinical Department of Laboratory Medicine of Jiangsu Province Hospital.
Funding: This study was supported by Natural Science Youth Foundation of China under grant (No. 81501817) and Natural Science Youth Foundation of Jiangsu Province under grant (No. BK20151029).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.05.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 2006;9:245-53. [Crossref] [PubMed]
- Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology 1971;61:185-8. [PubMed]
- Miki K, Ichinose M, Shimizu A, et al. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn 1987;22:133-41. [PubMed]
- Copps J, Murphy RF, Lovas S. The production and role of gastrin-17 and gastrin-17-gly in gastrointestinal cancers. Protein Pept Lett 2009;16:1504-18. [Crossref] [PubMed]
- Tong W, Ye F, He L, et al. Serum biomarker panels for diagnosis of gastric cancer. Onco Targets Ther 2016;9:2455-63. [PubMed]
- Sun LP, Gong YH, Wang L, et al. Serum pepsinogen levels and their influencing factors: a population-based study in 6990 Chinese from North China. World J Gastroenterol 2007;13:6562-7. [Crossref] [PubMed]
- Agréus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol 2012;47:136-47. [Crossref] [PubMed]
- Lee JY, Kim N, Lee HS, et al. Correlations among endoscopic, histologic and serologic diagnoses for the assessment of atrophic gastritis. J Cancer Prev 2014;19:47-55. [Crossref] [PubMed]
- Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 2005;54:764-8. [Crossref] [PubMed]
- Zoalfaghari A, Aletaha N, Roushan N, et al. Accuracy of pepsinogens for early diagnosis of atrophic gastritis and gastric cancer in Iranian population. Med J Islam Repub Iran 2014;28:150. [PubMed]
- Di Mario F, Moussa AM, Cavallaro LG, et al. Clinical usefulness of serum pepsinogen II in the management of Helicobacter pylori infection. Digestion 2004;70:167-72. [Crossref] [PubMed]
- Hosokawa O. Can a serological test for selecting high-risk groups before endoscopy reduce gastric cancer deaths? Gastric Cancer 2013;16:280-1. [Crossref] [PubMed]
Cite this article as: Lou JF, Chen X, Zhang JX, Zhang Y. Discrepant impacts of age and gender factors on serum pepsinogens and gastrin-17 levels. J Lab Precis Med 2017;2:42.


