Hypothyroidism induced by propylthiouracil decrease sirtuin1 content in rat heart
Introduction
Silent information regulator 2 (SIR2) proteins are a family of class III histone deacetylases that are highly conserved in mammalian species. Seven subtypes (sirtuin1–7) have been identified (1-3). Among them, sirtuin1 (SIRT1) is highly expressed in mammalian hearts and can regulate a wide variety of cellular processes such as apoptosis, survival, DNA repair, and metabolism (4-6). Previous studies reported that SIRT1 can protect the myocardium through multiple pathways (7-9). The mitochondrial pathway has been widely examined. These studies indicated that SIRT1 can promote the anti-apoptotic effect of the mitochondria in myocardial cells by regulating the permeability, synthesis, and morphology of mitochondria (7-10). Decreased SIRT1 levels may have adverse effects on the myocardium such as increased myocardial oxidative stress (11). Previous studies also showed that SIRT1 was significantly reduced in patients with heart failure (11,12). These studies indirectly suggest that SIRT1 has protective effects on the myocardium, which may affect thyroid hormones.
Thyroid hormone is secreted by the thyroid gland, has been confirmed to play a crucial role in regulating myocardial metabolism, and is related to the myocardium and SIRT1 (13). Thyroxine can inhibit cardiomyocyte apoptosis, particularly in patients with ischemia-reperfusion injury, and thyroid hormones exert their protection effects mainly through the mitochondria (14). Previous studies showed that the levels of circulating thyroid hormones are decreased in patients with acute myocardial infarction (15) and pretreatment with thyroid hormones significantly alleviates myocardial ischemia-reperfusion injury (14). Therefore, appropriate thyroxine levels may have protective effects on the myocardium, and low thyroxine levels do not contribute to myocardial viability. However, studies found that hypothyroidism (HT) levels are high in patients with clinical coronary heart disease (16) and can lead to various disorders such as cardiac dysfunction and heart failure (17). Recently published clinical studies have suggested that HT is associated with increased all-cause and cardiovascular mortality (18). In addition, decreased thyroxine and SIRT1 are harmful to the myocardium.
The effect of HT on SIRT1 content in myocardial cells remains unknown. Therefore, the purpose of this study was to determine the effects of HT on the content of SIRT1 in myocardial cells of rats.
Methods
Animals and treatments
We used 20 6-week-old (adults) male Wistar rats with body weights (BWs) of 180–200 g. All rats were obtained from the Animal Center of the Academy of Military Medical Sciences (Beijing, China). All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH, publication number 85-23, revised 1996, http://grants.nih.gov/grants/olaw/olaw.htm). All protocols were approved by the Animal Subjects Committee of the Academy of Military Medical Sciences, Beijing, China, and the study was approved the Ethics committee of The Second People’s Hospital of Yichang. Hypothyroid rats were prepared according to a previous study (19). Briefly, the rats were housed in cages and maintained at 22–24 °C with a normal 12-h/12-h light-dark cycle. The rats had free access to chow and water. The rats were randomly divided into control groups or HT groups to receive the diet and the following drug regimens for 8 weeks: (I) control groups (n=10): 0.9% sodium chloride solution was administered via gavage at 1 mL/day; (II) HT groups (n=10): 0.05% PTU saline solution was administered via gavage at 1 mL/day.
Measure of triiodothyronine (TT3), tetraiodothyronine (TT4) and thyroid-stimulating hormone (TSH)
We obtained blood samples (1 mL) from the femoral artery of rats before intervention to measure the baseline levels of TT3, TT4, and TSH. The rats were weighed using standard methods and then euthanized by cervical dislocation after receiving intervention for 8 weeks. We collected blood samples (1 mL) from each rat by cardiac puncture after opening the chest. These blood samples were centrifuged at 12,000 rpm and 4 °C for 10 min. The supernatant was collected to determine TT3, TT4, and TSH levels using commercial RIA kits (BNIBT, A01TFB for TT3, A02TFB for TT4, A05FZB for TSH, Beijing, China) in accordance with the manufacturer instructions.
Protein extract and measurement
After 8 weeks of intervention, blood samples were collected and the heart was excised immediately. The hearts were rinsed with cold (4 °C) normal saline, dried with filter paper, and measured to determine the heart weight (HW) index (HWI, HW/BW). Next, the left ventricular tissue was isolated, placed on ice immediately, and homogenized in radio-immunoprecipitation assay lysis buffer (CWbio, Beijing, China). Total protein in the supernatant was extracted from the homogenate by centrifugation (12,000 rpm, 4 °C, 10 min). Quantification of total protein was conducted using the BCA method with a BCA Kit (Takara, Shiga, Japan).
Western blot analysis to determine relative SIRT1 content
For direct immunoblotting, aliquots of the lysate were mixed with 5× SDS-PAGE sample loading buffer (containing 5% 2-mercaptoethanol) and boiled for 10 min. The same amounts of proteins (60 µg) were loaded into 10% acrylamide gels and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The PVDF membranes were blocked with 8% nonfat milk in Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBST) for 1 h at room temperature (about 25 °C) and then incubated overnight with an anti-SIRT1 antibody (1:8,000, ab110304; Abcam, Cambridge, UK) and anti-β-actin (1:1,000, ab8226; Abcam) at 4 °C. The PVDF membranes were washed extensively with TBST before incubation for 1 h with a secondary anti-mouse or anti-rabbit IgG (1:1,000, Cell Signaling Technology, Inc., Danvers, MA, USA) conjugated to horseradish peroxidase. Protein bands were detected by the standard enhanced chemiluminescence method, and images were digitized. Next, relative band intensities were measured by densitometry using Image Lab software version 4.1.0 (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data were analyzed with SPSS16.0 (SPSS, Inc., Chicago, IL, USA). Data from independent experiments were expressed as the mean ± SD of at least three experiments. Differences of continuous variables between the two groups were compared by analysis of variance with repeated measures and the paired t-test was used to compare differences before and after intervention. The Spearman approach was used to analyze the correlation. P<0.05 was considered statistically significant.
Results
Effects of PTU on thyroid function in rats
PTU has a significant effect on the thyroid function of rats. No statistical differences were found between the two groups in TT3, TT4, and TSH before intervention. After intervention for 8 weeks, serum TT3 and TT4 concentrations were significantly lower than those before intervention and in the control groups (P<0.05), while serum TSH levels were higher than those before intervention and in the control groups (P<0.05) (Figure 1).

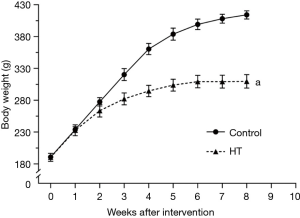
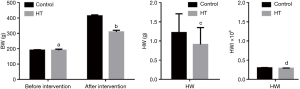
Effects of PTU on body and HW of rats
At baseline, BW was not significantly different between the HT groups and control groups (P>0.05) (Figure 2). HT induced by PTU resulted in significant slowing of BW gain compared to rats in the control groups (P<0.05) (Figures 2,3). Interestingly, the HW of rats in HT groups was significantly lower than that in the control group (P<0.05) (Figure 2), but there was no significant difference in HWI between the two groups (P>0.05) (Figure 2).
Effects of PTU on SIRT1 of myocardium of rats
The SIRT1 content of the myocardium in the PTU groups was lower than that in the control groups (P<0.05) (Figure 4).

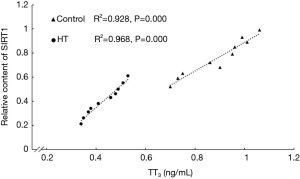
Correlation between relative SIRT1 content and thyroid hormone concentrations
The results of correlation analysis showed that the relative content of SIRT1 was correlated with thyroid hormones levels, particularly for TT3 (P<0.05) (Figure 5).
Discussion
A previous study showed that HT had a promoting effect on SIRT2 (homologous protein of SIRT1) in nerve cells (20). However, the effect of HT on SIRT1 in the myocardium remained unclear. We evaluated the relationship between HT induced by PTU and SIRT1 content in rat hearts. The myocardial SIRT1 content of rats decreased significantly in HT. The rats showed slower weight gain and lower HW in HT. These results are consistent with those of previous studies (21,22).
Sirtuins are analogs of SIR2 and include seven subtypes (SIRT1–7) that exist as the oxidized form of nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase (1-3). A relationship between thyroid hormones and SIRT1 was observed in the present study. Previous studies have explored the relationship between thyroid hormones and SIRT1 in epidermal keratinocytes and hepatocytes, but not in the myocardium. A previous study showed that expression of SIRT1 in cultured human epidermal keratinocytes was increased after treatment with T3 (23). Diiodothyronine (T2) can rapidly increase the activity of SIRT1 in hepatocytes (24). Thyroid hormones increase the content of SIRT1, and SIRT1 may exert negative feedback regulation on thyroid hormones. In obese rats induced by diet, the suppression of hypothalamic SIRT1 significantly stimulates the hypothalamus-pituitary-thyroid axis and promotes the secretion of thyroid hormones (25). The mechanism of the decrease of SIRT1 after HT remains unclear. Additionally, how thyroid hormones increase SIRT1 content is unclear. SIRT1 has been shown to participate in myocardial protection. First, SIRT1 can increase the resistance to oxidative stress and ischemia/reperfusion injury via multiple pathways (4,26,27). Decreased SIRT1 in the heart may aggravate myocardial ischemia-reperfusion injury (28), cardiomyocyte apoptosis, and early-onset heart failure (29). Second, SIRT1 plays an important role in anti-atherosclerotic lesions (30) and reduces the oxidation of low-density lipoprotein (31). Third, SIRT1 can inhibit myocardial apoptosis by regulating the mitochondria in multiple manner (9,10,32-35). Therefore, SIRT1 is a myocardial protective factor, and its reduction adversely affects the myocardium. These studies demonstrated that HT plays an important role in heart failure, myocardial infarction, and other myocardial ischemia. The present study found that administration of thyroid hormone may improve myocardial ischemia and hypoxia as well as apoptosis by reducing HT and SIRT1. Our study also suggests that TH-induced atherosclerosis is associated with a reduction in SIRT1 by HT.
There were some limitations to our study. First, we did not observe myocardial tissue sections to determine whether there is a significant difference in morphology of myocardial cells between the two groups. Second, this study did not explore whether HT induced by PTU reduces the expression of SIRT1 at the transcriptional level. Finally, animal models were used, and we did not validate the results in cultured cells. Further studies are needed to examine the morphological changes in myocardial cells in the reduction of SIRT1 induced by HT and the effect of HT on the transcription of SIRT1. Whether increasing the content of SIRT1 or promoting the activity of SIRT1 in the myocardium can reduce myocardial damage caused by HT and whether this contributes to the protective effect of SIRT1 by increasing thyroid hormones requires further analysis. However, this study confirmed that PTU-induced HT leads to decreased levels of SIRT1 in cardiomyocytes, and this decrease was related to decreased TT3. Based on our results, myocardium injury caused by HT may be associated with reduced SIRT1 in the heart.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation (No. 81560552).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.08.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics committee of The Second People’s Hospital of Yichang (approval ID: 201600225), in compliance with national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Onyango P, Celic I, McCaffery JM, et al. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A 2002;99:13653-8. [Crossref] [PubMed]
- Kyrylenko S, Kyrylenko O, Suuronen T, et al. Differential regulation of the Sir2 histone deacetylase gene family by inhibitors of class I and II histone deacetylases. Cell Mol Life Sci 2003;60:1990-7. [Crossref] [PubMed]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem 2004;73:417-35. [Crossref] [PubMed]
- Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 2007;100:1512-21. [Crossref] [PubMed]
- Khan AN, Lewis PN. Use of substrate analogs and mutagenesis to study substrate binding and catalysis in the Sir2 family of NAD-dependent protein deacetylases. J Biol Chem 2006;281:11702-11. [Crossref] [PubMed]
- Zhong L, Mostoslavsky R. Fine tuning our cellular factories: sirtuins in mitochondrial biology. Cell Metab 2011;13:621-6. [Crossref] [PubMed]
- Karch J, Molkentin JD. Is p53 the long-sought molecular trigger for cyclophilin D-regulated mitochondrial permeability transition pore formation and necrosis. Circ Res 2012;111:1258-60. [Crossref] [PubMed]
- Thakur BK, Dittrich T, Chandra P, et al. Involvement of p53 in the cytotoxic activity of the NAMPT inhibitor FK866 in myeloid leukemic cells. Int J Cancer 2013;132:766-74. [Crossref] [PubMed]
- Huajie Z. Effect of resveratrol on myocardial energy metabolism in sepsis rats. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:980-3. [PubMed]
- Menzies KJ, Hood DA. The role of SirT1 in muscle mitochondrial turnover. Mitochondrion 2012;12:5-13. [Crossref] [PubMed]
- Hsu CP, Zhai P, Yamamoto T, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010;122:2170-82. [Crossref] [PubMed]
- Lu TM, Tsai JY, Chen YC, et al. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci 2014;21:57. [Crossref] [PubMed]
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501-9. [Crossref] [PubMed]
- Forini F, Nicolini G, Iervasi G. Mitochondria as key targets of cardioprotection in cardiac ischemic disease: role of thyroid hormone triiodothyronine. Int J Mol Sci 2015;16:6312-36. [Crossref] [PubMed]
- Franklyn JA, Gammage MD, Ramsden DB, et al. Thyroid status in patients after acute myocardial infarction. Clin Sci (Lond) 1984;67:585-90. [Crossref] [PubMed]
- Mayer O, Simon J, Filipovský J, et al. Hypothyroidism in coronary heart disease and its relation to selected risk factors. Vasc Health Risk Manag 2006;2:499-506. [Crossref] [PubMed]
- Tang YD, Kuzman JA, Said S, et al. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation 2005;112:3122-30. [Crossref] [PubMed]
- Komatsu R, You J, Mascha EJ, et al. The effect of hypothyroidism on a composite of mortality, cardiovascular and wound complications after noncardiac surgery: a retrospective cohort analysis. Anesth Analg 2015;121:716-26. [Crossref] [PubMed]
- Cernohorský J, Kolár F, Pelouch V, et al. Thyroid control of sarcolemmal Na+/Ca2+ exchanger and SR Ca2+-ATPase in developing rat heart. Am J Physiol 1998;275:H264-73. [PubMed]
- Weltman NY, Ojamaa K, Savinova OV, et al. Restoration of cardiac tissue thyroid hormone status in experimental hypothyroidism: a dose-response study in female rats. Endocrinology 2013;154:2542-52. [Crossref] [PubMed]
- Liu Z, Gerdes AM. Influence of hypothyroidism and the reversal of hypothyroidism on hemodynamics and cell size in the adult rat heart. J Mol Cell Cardiol 1990;22:1339-48. [Crossref] [PubMed]
- Bagchi N, Brown TR, Schneider DS, et al. Effect of amiodarone on rat heart myosin isoenzymes. Circ Res 1987;60:621-5. [Crossref] [PubMed]
- Vidali S, Chéret J, Giesen M, et al. Thyroid hormones enhance mitochondrial function in human epidermis. J Invest Dermatol 2016;136:2003-12. [Crossref] [PubMed]
- de Lange P, Cioffi F, Senese R, et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes 2011;60:2730-9. [Crossref] [PubMed]
- Cyr NE, Steger JS, Toorie AM, et al. Central Sirt1 regulates body weight and energy expenditure along with the POMC-derived peptide α-MSH and the processing enzyme CPE production in diet-induced obese male rats. Endocrinology 2015;156:961-74. [Crossref] [PubMed]
- Kume S, Haneda M, Kanasaki K, et al. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic Biol Med 2006;40:2175-82. [Crossref] [PubMed]
- Ding M, Lei J, Han H, et al. SIRT1 protects against myocardial ischemia-reperfusion injury via activating eNOS in diabetic rats. Cardiovasc Diabetol 2015;14:143. [Crossref] [PubMed]
- Yu L, Liang H, Dong X, et al. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res 2015;59:376-90. [Crossref] [PubMed]
- Mu W, Zhang Q, Tang X, et al. Overexpression of a dominant-negative mutant of SIRT1 in mouse heart causes cardiomyocyte apoptosis and early-onset heart failure. Sci China Life Sci 2014;57:915-24. [Crossref] [PubMed]
- Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle 2011;10:640-7. [Crossref] [PubMed]
- Zhang QJ, Wang Z, Chen HZ, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 2008;80:191-9. [Crossref] [PubMed]
- Itoh T, Kouzu H, Miki T, et al. Cytoprotective regulation of the mitochondrial permeability transition pore is impaired in type 2 diabetic Goto-Kakizaki rat hearts. J Mol Cell Cardiol 2012;53:870-9. [Crossref] [PubMed]
- Li P, Meng X, Bian H, et al. Activation of sirtuin 1/3 improves vascular hyporeactivity in severe hemorrhagic shock by alleviation of mitochondrial damage. Oncotarget 2015;6:36998-7011. [Crossref] [PubMed]
- Ye L, Yang S. Sirtuin 3 inhibits cardiomyocyte apoptosis by reducing cytochrome C release in myocardiac H9c2 cells with calcium overload. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015;31:1031-5. [PubMed]
- Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 2008;105:3374-9. [Crossref] [PubMed]
Cite this article as: Xu QY, Wang XL, Peng YF. Hypothyroidism induced by propylthiouracil decrease sirtuin1 content in rat heart. J Lab Precis Med 2017;2:67.