
Can urinary exosomal micro-RNA detection become a diagnostic and prognostic gold standard for patients with lupus nephritis and diabetic nephropathy?
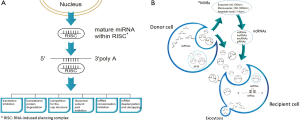
In 1993, Lee et al. (1) observed that lin-4 gene could control the embryonic development of C. elegans, but failed to encode a protein molecule. Besides, the gene transcribes a pair of microRNAs (miRNAs). The short RNA is approximately 22 nucleotides (nt) in length whereas the long one is 61 nt that was predicted to fold a stem loop with anti-sense complementary to 3'-untranslated region (UTR) of lin-14 messenger RNA (mRNA) (2). In mammalian genomes, these miRNA genes exist as either independent transcription units or are located in the introns of other genes (3). Nowadays, it is conceivable that miRNA is one of the most diverse spectrum of non-coding (nc) RNAs consisting of more than 2,000 different species (4). The crucial function of these miRNAs involves the regulation of mRNA expression through interference with the 3'-UTR. It is estimated that more than 60% of mammalian genes are regulated by these intracellular miRNAs (5). Furthermore, a pool of cell-free miRNAs is found in the biological fluids including plasma, milk, tears, saliva, urine, amniotic fluid, cerebrospinal fluid, semen and ascites (6). This may imply that these extracellular miRNAs act potentially as signaling molecules for cell-cell communication (7). More recent investigations have revealed that these extracellular miRNAs exist in three forms, i.e., exosomes (40–100 nm), microvesicles (100–1,000 nm) and apoptotic bodies (1–4 µm) as shown in Figure 1. Exosomes are exocytotic membrane-derived vesicles that contain a variety of proteins, nucleic acids and lipids for mediating cell-cell communication via proteins and miRNAs delivery (8). Since the amount and composition of exosomal miRNAs are found different among diseases and healthy individuals, they can potentially become novel biomarkers for clinical applications (9).
Pathophysiology of chronic kidney diseases (CKDs) and its correlation to biochemical/immunological biomarkers currently available
CKDs are caused basically by immunological and non-immunological mechanisms. Lupus nephritis (LN) is a typical autoimmune-mediated CKD caused by multiple immunological responses. The immunopathological findings of renal tissues in LN include immune complex deposition with complement activation, glomerular crescents, podocyte injury, inflammatory cell infiltration, vascular injury, and tubulointerstitial lesions (10). In contrast, diabetic nephropathy (DN) is one of the most common microvascular complications of diabetes mellitus (DM) and a representative non-immunologic-mediated CKD. The characteristic pathologic features of DN include thickened glomerular basement membrane, increased extracellular matrix formation, podocyte loss, epithelial-mesenchymal transition (EMT) and renal cell apoptosis (11). However, some immunological components such as increased innate immune receptors of Toll-like receptor 2 and 4 (12) and activation of the complement system (13) are also implicated in DN. In clinical practice, kidney biopsy remains the gold standard for differential diagnosis and treatment decision for various CKDs. However, the biopsy procedure is rather invasive with complication risks. Instead, the non-invasive biomarkers obtained from serum or urine specimens have been extensively investigated by authors for predicting, diagnosing and prognostic purposes. Hsieh et al. (14) reviewed the literature and concluded that currently reported serum or urine biomarkers for LN are exclusively inflammatory or immune-related molecules that are unable to reflect the ongoing scenario of pathological changes in LN. The similar impasse is also encountered in DN where urine-specific miRs have been found positively correlated to the albuminuria and TGF-β1 levels, but not comparable with the gold-standard histological findings (15).
Urinary exosomal miRNAs are potential biomarkers for LN and DN
The physiologic functions of extracellular vesicles in normal renal glomeruli include immune modulation, cell proliferation, tissue regeneration, matrix synthesis, and cell-cell communication (16). Jia et al. (15) and Eissa et al. (17) have found that increased urinary exosomal miR-192, miR-156, miR-34a and miR-636 are correlated to albuminuria/proteinuria in patients with DN. In addition, diverse urinary exosomal proteins, lipids, mRNAs, mitochondrial DNAs and miRNAs have been reported in different acute kidney diseases, CKDs and DN by Bhatt et al. (18) and Street et al. (19). Recently, Cardenas-Gonzalez et al. (20) profiled 2401 urinary exosomal miRNAs by identifying, confirming and replicating them in patients with LN and DN in two-run cohort study. They conclusively clarified some urinary exosomal miRNAs as potential sensitive and specific urine biomarkers after correlating them with renal functions and histopathology of the biopsy-proven CKDs. They demonstrated down-regulation of miR-3201 and miR-1273e in correlation with endocapillary glomerular inflammation in LN. In addition, down-regulation of miR-2861, miR-1915-3P and miR-4532 were correlated to decreased serum estimated glomerular filtration rate (eGFR), renal interstitial fibrosis and tubular atrophy in DN. The authors proposed that these novel urinary exosomal miRNAs could be regarded as liquid biopsy in patients with LN and DN. Table 1 summarizes the correlation of urinary exosomal miRNAs with histological changes or functional impairments in LN and DN in the literature.
Table 1
| Exosomal miRNA expression | Correlation with |
|---|---|
| Lupus nephritis | |
| Increased expression | Glomerulonephritis (14) |
| miR-125a | |
| miR-150 | |
| miR-155 | |
| miR-146 | |
| Decreased expression | |
| miR-141 | |
| miR-192 | |
| miR-200a | |
| miR-200c | |
| miR-221 | |
| miR-222 | |
| miR-429 | |
| Decreased expression | Endocapillary glomerular inflammation (20) |
| miR-3201 | |
| miR-1273e | |
| Diabetic nephropathy | |
| Increased expression | Proteinuria albuminuria (16) |
| miR-130a | |
| miR-145 | |
| miR-15b | |
| miR-34a | |
| miR-192 | |
| Decreased expression | |
| miR-155 | |
| miR-424 | |
| Decreased expression | Decrease in eGFR interstitial fibrosis tubular atrophy (20) |
| miR-2861 | |
| miR-1915-3p | |
| miR-4532 |
miR, microRNA; eGFR, estimated glomerular filtration rate.
A likelihood of miRNAs in urine to be biomarkers for LN and DN
A clinically useful disease biomarker should fulfill the criteria of high sensitivity, high specificity, general availability and reasonable cost for predicting, monitoring and/or foreseeing the outcome of the disease. More than 2,000 miRNomes are now available for the detection of global urinary miRNAs. It is believed that CKD jeopardizes patients to the occurrence of cardiovascular diseases, end-stage renal diseases and mortality (21). To directly reflect the renal histopathologic changes, the detection of excreted molecules by diseased kidney in the urine seems feasible and practical. Exosomes are small extracellular vesicles carrying proteins and nucleic acids that can be transferred in active form to nearby or distant tissues for cell-cell communication. In patients with DM, blood glucose homeostasis requires a constant communication between insulin-secreting and insulin-sensitive cells. In addition to endocrine modulation, exosomes released from pancreatic beta cells, skeletal muscle, adipose tissue and liver can serve as new players in metabolic organ cross-talk (22). In case of deranged exosomal miRNA cross-talk among these metabolic organs, in-time blood glucose regulation cannot be achieved, leading to DM. The high blood glucose level subsequently elicits glomerular podocyte dysfunction and depletion through an EMT together with growth hormone-induced podocyte apoptosis in DN (23). Similarly, the autoimmune-induced inflammation in LN may also cause podocyte injury as reported by Yu et al. (10). It remains unclear why only some particular exosomal miRNA biogenesis decreases in LN and DN as reported by Cardenas-Gonzalez et al. (20). The sophisticated interactions among these aberrant miRNAs in LN and DN are still open questions. Undoubtedly, these perplexities need more investigations to clarify. In addition to exosomal miRNAs, it is quite interesting that long nc RNAs (lncRNAs) and short nc RNAs (sncRNAs) with molecular weight of approximately 200-nt in length are also participants in transcriptomic regulation of immune cells and autoimmune diseases (24). These larger ncRNAs were found more specific in regulating gene expression than the multi-targeting miRNAs (24). It is of equal interesting to know whether or not these lncRNAs and sncRNAs appear in urinary exosomes for cell-cell communications in CKDs.
Conclusions
Transcriptomics and bioinformatics have discovered the expression of thousands of ncRNAs which are functional RNAs for regulating mRNAs expression. These ncRNAs may serve as potential biomarkers for diagnosis, monitoring, or prognosis of patients with DN or LN via so-called “liquid biopsy” from urine or other body fluids instead of invasive tissue biopsy. A simple assay kits for detecting urinary exosomal ncRNAs are expected to be developed for the “precision medicine” in the management of DN and LN instead of the potentially hazardous renal biopsy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Ming-Zhu Gao, MD (Department of Laboratory Medicine, Wuxi No.2 People’s Hospital of Nanjing Medical University, Wuxi, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.06.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochromic gene lin-4 encodes small RNAs with antisense complementary to lin-14. Cell 1993;75:843-54. [Crossref] [PubMed]
- Wightman B, Ha I, Ruvkun G. Post transcriptional regulation of the heterchromic gene lin-14 by lin-4 mediated temporal pattern formation in C. elegans. Cell 1993;75:855-62. [Crossref] [PubMed]
- Rodriguez A, Griffiths-Jones S, Ashurst JL, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res 2004;14:1902-10. [Crossref] [PubMed]
- Friedländer MR, Lizano E, Houben AJ, et al. Evidence for the biogenesis of more than 1000 novel human microRNAs. Genome Biol 2014;15:R57. [Crossref] [PubMed]
- Friedman RC, Fárh KK, Burge CB, et al. Most mammalian mRNAs are conserved targets of miRNAs. Genome Res 2009;19:92-105. [Crossref] [PubMed]
- Weber JA, Baster DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem 2010;56:1733-41. [Crossref] [PubMed]
- Makarova JA, Shkurnikov MU, Wicklein D, et al. Intracellular and extracellular microRNA: an update on localization and biological role. Prog Histochem Cytochem 2016;51:33-49. [Crossref] [PubMed]
- Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 2015;13:17-24. [Crossref] [PubMed]
- Lin J, Li J, Huang B, et al. Exosomes: novel biomarkers for clinical diagnosis. ScientificWorldJournal 2015;2015:657086.
- Yu F, Haas M, Glassock R, et al. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol 2017;13:483-95. [Crossref] [PubMed]
- Han Q, Zhu H, Chen X, et al. Non-genetic mechanisms of diabetic nephropathy. Front Med 2017;11:319-32. [Crossref] [PubMed]
- Mudaliar H, Pollock C, Panchapakesan U. Role of Toll-like receptors in diabetic nephropathy. Clin Sci (Lond) 2014;126:685-94. [Crossref] [PubMed]
- Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol 2010;6:94-101. [Crossref] [PubMed]
- Hsieh SC, Tsai CY, Yu CL. Potential serum and urine biomarkers in patients with lupus nephritis and the unsolved problems. Open Access Rheumatol 2016;8:81-91. [Crossref] [PubMed]
- Jia Y, Guan M, Zheng Z, et al. miRNAs in urine extracellular vesicles as predictors of early-stage diabetic nephropathy. J Diabetes Res 2016;2016:7932765.
- Trionfini P, Benigni A. MicroRNAs as master regulation of glomerular functions in health and disease. J Am Soc Nephrol 2017;28:1686-96. [Crossref] [PubMed]
- Eissa S, Matoboli M, Aboushahba R, et al. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetes kidney disease. J Diabetes Complications 2016;30:1585-92. [Crossref] [PubMed]
- Bhatt K, Kato M, Natarajan R. Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol (Renal Physiol) 2016;310:F109-18. [PubMed]
- Street JM, Koritzinsky EH, Glispie DM, et al. Urine exosomes: an emerging trove of biomarkers. Adv Clin Chem 2017;78:103-22. [Crossref] [PubMed]
- Cardenas-Gonzalez M, Srivastava A, Pavkovic M, et al. Identification, Confirmation, and Replication of Novel Urinary MicroRNA Biomarkers in Lupus Nephritis and Diabetic Nephropathy. Clin Chem 2017;63:1515-26. [Crossref] [PubMed]
- Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms and therapeutic approaches. J Am Soc Nephrol 2012;23:1929-39. [Crossref] [PubMed]
- Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab 2017;19:137-46. [Crossref] [PubMed]
- Anil Kumar P, Welsh GI, Saleem MA, et al. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 2014;5:151. [Crossref] [PubMed]
- Lai NS, Yu CL, Lu MC. Immunopathogenesis of systemic lupus erythematosus and rheumatoid arthritis: the role of aberrant expression of non-coding RNSs and T cells. Clin Exp Immunol 2017;187:327-36. [Crossref] [PubMed]
Cite this article as: Tsai CY, Lu MC, Yu CL. Can urinary exosomal micro-RNA detection become a diagnostic and prognostic gold standard for patients with lupus nephritis and diabetic nephropathy? J Lab Precis Med 2017;2:91.


