
Middle-distance running and DNA damage in diabetics
Introduction
Physical activity is now regarded as an essential part in prevention and therapeutic management of subjects with type 1 and 2 diabetes mellitus, since it directly contribute to generate a kaleidoscope of metabolic benefits such as optimized glucose utilization and control of both blood lipids and blood pressure, among others (1-3). Nevertheless, some doubts remain about the optimal balance between type, intensity and duration of physical exercise, thus hampering the recognition of exercise patterns which would then translate into the largest possible benefits for subjects at risk of, or already diagnosed with, diabetes. This is especially important since diabetic subjects frequently have many co-morbidities (e.g., dyslipidemia, obesity, hypertension, impaired renal function), which can ultimate contribute to jeopardize their health status while performing unsuitable forms or inappropriate intensities of physical exercise. The potential impact of physical exercise on DNA integrity is an additional issue. Previous studies showed that high-volume exercise, such as that characterizing triathlon and middle- to long-distance running or cycling, may generate a certain amount of transitory DNA damage (4). Evidence has also been provided that an enhanced oxidative stress is a hallmark of diabetes and may contribute to trigger an endogenous oxidative DNA injury, resulting in enhanced susceptibility to carcinogens, to a lower efficacy of DNA repair (5,6), and to a greater propensity to develop the most typical diabetic complications (7).
Therefore, the aim of the present study was to investigate the burden of DNA injury after middle-distance endurance running in diabetic subjects in comparison with a population of euglycemic subjects.
Methods
Study design
This study was part of a specific event called “Run For Science”, organized under the auspices of the University of Verona (Italy) in the year 2015. Nineteen euglycemic amateur runners and [2 women and 17 men; median age 41 years and interquartile range (IQR), 34–44 years] and 16 diabetic amateur runners (9 with type 1 and 7 with type 2 diabetes; 5 women and 11 men; median age 53 years and IQR, 45–63 years) were engaged in a 21.1 km running trial, completed under competing conditions. The run started at 7:00 AM and developed on a nearly flat circuit (maximal slope, 1.8%), as more comprehensively described elsewhere (8). All athletes belong to a non-professional running team and were ordinarily engaged in recreational running at least twice a week. All subjects were asked to abstain from eating food and performing demanding physical exercise in the 8 and 48 hours before the start of the trial, respectively. Venous blood was collected into evacuated blood tubes containing K2EDTA (Vacutest Kima s.r.l., Padova, Italy) by venipuncture of an antecubital vein, immediately before the start of the run and 3 hours after the athletes had crossed the finishing line. All specimens were accurately mixed after collection and were then shipped to the laboratory of clinical chemistry and hematology of the University Hospital of Verona (Italy) for being analyzed. The study was carried out in accordance with the Declaration of Helsinki and was approved by the University Hospital of Verona Institutional Review Board (n. 165038). Informed consent was obtained from all subjects.
Analysis of γ-H2AX foci
The analysis of DNA injury was carried by means of phosphorylated histone protein H2AX (γ-H2AX) foci assessment on lymphocytes (AKLIDES Nuk Human Lymphocyte Complete, Medipan, Blankenfelde-Mahlow, Germany). The full details of this technique have been earlier published (9,10). The final assessment of γ-H2AX foci was completed with a pattern recognition algorithm implemented in the automated fluorescence interpretation system AKLIDES (Medipan GMBH, Dahlewitz/Berlin, Germany). A minimum number of 100 cells were evaluated for each sample, and the following parameters were finally estimated: (I) relative number (i.e., percentage) of cells displaying γ-H2AX foci; (II), mean number of γ-H2AX foci detected per single cell; (III) overall number of γ-H2AX foci detected in all cells. All parameters were analyzed in duplicate slides and final data were reported as average of two measures. The variation of γ-H2AX foci parameters was expressed as absolute difference of values obtained 3 hours after the end of the trial and values obtained at rest [i.e., (post-run values) − (pre-run values)]. The expression of changes in terms of “ratio” was unfeasible, since the value at rest was zero in several cases.
Statistical analysis
Final results were reported as median and IQR. The significance of differences was evaluated with Wilcoxon’s test for paired samples or Mann-Whitney U test, when appropriate. The level of statistical significance was set at P<0.05, and statistical analysis was carried out with Analyse-it (Analyse-it Software Ltd, Leeds, UK).
Results
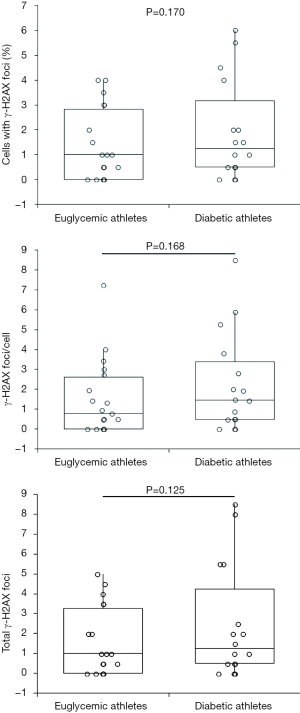
The age of the euglycemic athletes was lower than that of the diabetic athletes (P<0.001), whilst type 2 diabetics (67 years; IQR, 62–73 years) were older than both type 1 diabetics (45 years; IQR 42–47 years; P<0.001) and euglycemic athletes (41 years; IQR, 34–44 years; P<0.001). No significant difference was found between the age of euglycemic and type 1 diabetic athletes (P=0.052). The values of γ-H2AX foci parameters at rest were found to be substantially similar in diabetic and euglycemic subjects (Figure 1). Although type 2 diabetics displayed a trend toward higher values of all γ-H2AX foci parameters at rest compared to type 1 diabetics, in no case such difference reached statistical significance (cells with γ-H2AX foci: 1.00% versus 2.00%, P=0.061; γ-H2AX foci/cell: 0.88 versus 1.93, P=0.065; total γ-H2AX foci: 1.00 versus 2.50, P=0.058). In multivariate linear regression analysis, in which each of the γ-H2AX foci parameter was entered as dependent variable whereas age and diabetic status (euglycemic, type 1 and type 2 diabetes) were entered as depend variables, cells with γ-H2AX foci, γ-H2AX foci/cell and total γ-H2AX foci were not found to be significantly associated with both glycaemic status and age (all interactions, P>0.25).
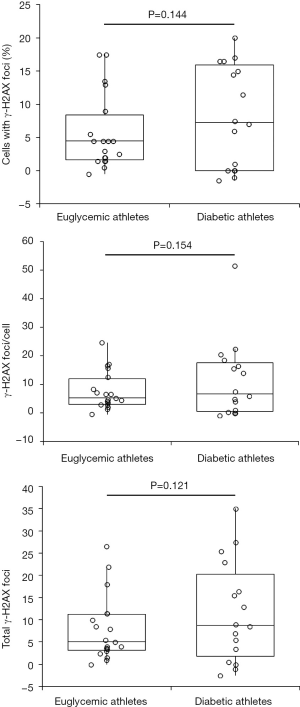
In accordance with previous data (4), a substantial increase of all γ-H2AX foci parameters was observed after the 21.1 km running trial, both in euglycemic and diabetic subjects. In euglycemic athletes, cells with γ-H2AX foci increased by 4.50% (IQR, 1.75–7.25%; P=0.001), γ-H2AX foci/cell increased by 5.26% (IQR, 3.26–10.53%; P<0.001) and total γ-H2AX foci increased by 5.00% (IQR, 3.25–10.75%; P<0.001), whilst in diabetic athletes cells with γ-H2AX foci increased by 7.25% (IQR, 0–15.38%; P=0.001), γ-H2AX foci/cell increased by 6.71% (IQR, 0.79–16.92%; P=0.002) and total γ-H2AX foci increased by 8.75% (IQR, 2.75–18.13%; P<0.001). Notably, the absolute increase of all γ-H2AX foci parameters was found to be non-significantly different between the two study groups (Figure 2). Interestingly, the absolute increase of cells with γ-H2AX foci was found to be non-significantly higher in type 2 than in type 1 diabetics (15.0% versus 6.0%; P=0.082), whilst the values of γ-H2AX foci/cell (4.0 versus 18.5; P=0.005) and total γ-H2AX foci (16.5 versus 7.0; P=0.031) exhibited an absolute increase that was found to be significantly higher in type 2 than type 1 diabetics. However, such differences were no longer statistically significant after correcting the values of all γ-H2AX foci parameters for the age of the athletes (cells with γ-H2AX foci, P=0.280; γ-H2AX foci/cell, P=0.056; total γ-H2AX foci, P=0.127).
Discussion
The oxidative stress is one of the most important triggers of cell membrane injury and genomic damage. Its actual contribution to the pathogenesis of diabetes and its complication has been clearly established (11), along with its direct impact on genomic instability, through promoting the development of double-strand breaks (DSBs) (12). Automated analysis of γH2AX foci in human peripheral blood mononuclear cells by means of indirect immunofluorescence is now considered one of the most suitable and reliable approach for detecting DSBs and hence assessing DNA damage in heath and disease, an approach which has been successfully used for studying the extent of a variety of genomic damages caused by different environmental, chemical and physical sources (13-15).
Two previous studies showed that the burden of DSBs may be higher in peripheral blood mononuclear cells of diabetics than in those of euglycemic subjects. However, this evidence was only reported in a small study on adolescents with type 1 diabetes (16), and in an in vitro study using pancreatic β cells (17). An acute increase of DSBs, highly correlated with intensity and duration of the physical effort, has also been observed after endurance exercise in euglycemic subjects (4). It is hence conceivable that the combination of these two important sources of oxidative stress (i.e., impaired glucose metabolism and endurance exercise) may act synergistically to generate an even more unfavorable impact on DNA stability. Nevertheless, the results of our study seem to contradict this hypothesis, since we found that the all the γH2AX foci parameters were substantially similar in euglycemic and diabetics athletes at rest and that, even more importantly, their values were not correlated with either the age of the subjects or the diabetic status (Figure 1). Another important finding of our study is that an acute bulk of endurance exercise was indeed effective to enhance the burden of DSBs, but the absolute increase was generally comparable between euglycemic and diabetic athletes after adjustment for the age of the subjects (Figure 2). This is a quite reasonable evidence, since the burden of DSBs has been shown to increase in parallel with aging (18). It is also plausible that the acute increase of DSBs recorded after the running trial may only be transitory, and can then be efficiently counteracted by the many advantages that regular physical exercise could bring to both healthy and diabetic populations. Undoubtedly, the small sample size and the lack of additional data relative to the DBSs status during the recovery phase, are recognized limitations of the present study.
Conclusions
Taken together, the results of this original study show for the first time that the burden of DSBs in physically active type 1 and 2 diabetics at rest is comparable to that of a matched population of euglycemic recreational athletes and, even more importantly, the extent of DNA damage in diabetics engaged in middle-distance running is non-significantly different to that observed in euglycemic subjects. Regarding DNA injury, type and intensity of physical exercise characterizing middle-distance running can hence be considered as safe as for the euglycemic population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “DNA Damage Assessment for Precision Medicine”. The article has undergone external peer review.
Conflicts of Interest: The series “DNA Damage Assessment for Precision Medicine” was commissioned by the editorial office without any funding or sponsorship. Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Martina Montagnana serves as the unpaid Associate Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Dirk Roggenbuck served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from August 2017 to July 2019. D Roggenbuck is a shareholder of Medipan and has a managerial position at the company. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was carried out in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the University Hospital of Verona Institutional Review Board (n. 165038). Informed consent was obtained from all subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gill JM, Cooper AR. Physical activity and prevention of type 2 diabetes mellitus. Sports Med 2008;38:807-24. [Crossref] [PubMed]
- Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147-67. [Crossref] [PubMed]
- Zisser H, Gong P, Kelley CM, et al. Exercise and diabetes. Int J Clin Pract Suppl 2011;71-5. [Crossref] [PubMed]
- Danese E, Lippi G, Sanchis-Gomar F, et al. Physical Exercise and DNA Injury: Good or Evil? Adv Clin Chem 2017;81:193-230. [Crossref] [PubMed]
- Blasiak J, Arabski M, Krupa R, et al. DNA damage and repair in type 2 diabetes mellitus. Mutat Res 2004;554:297-304. [Crossref] [PubMed]
- Lee SC, Chan JC. Evidence for DNA damage as a biological link between diabetes and cancer. Chin Med J (Engl) 2015;128:1543-8. [Crossref] [PubMed]
- Hinokio Y, Suzuki S, Hirai M, et al. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia 1999;42:995-8. [Crossref] [PubMed]
- Lippi G, Schena F. Run for Science (R4S): the history of a successful project of precision and laboratory medicine in sport and exercise. J Lab Precis Med 2017;2:11. [Crossref]
- Runge R, Hiemann R, Wendisch M, et al. Fully automated interpretation of ionizing radiation-induced γH2AX foci by the novel pattern recognition system AKLIDES®. Int J Radiat Biol 2012;88:439-47. [Crossref] [PubMed]
- Lippi G, Buonocore R, Tarperi C, et al. DNA injury is acutely enhanced in response to increasing bulks of aerobic physical exercise. Clin Chim Acta 2016;460:146-51. [Crossref] [PubMed]
- Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J 2016;24:547-53. [Crossref] [PubMed]
- Kreuz S, Fischle W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016;8:843-62. [Crossref] [PubMed]
- Willitzki A, Lorenz S, Hiemann R, et al. Fully automated analysis of chemically induced γH2AX foci in human peripheral blood mononuclear cells by indirect immunofluorescence. Cytometry A 2013;83:1017-26. [Crossref] [PubMed]
- Rothkamm K, Barnard S, Moquet J, et al. DNA damage foci: Meaning and significance. Environ Mol Mutagen 2015;56:491-504. [Crossref] [PubMed]
- Reddig A, Rübe C, Rödiger S, et al. DNA damage assessment and potential applications in laboratory diagnostics and precision medicine. J Lab Precis Med 2018; In Press.
- Giovannini C, Piaggi S, Federico G, et al. High levels of γ-H2AX foci and cell membrane oxidation in adolescents with type 1 diabetes. Mutat Res 2014;770:128-35. [Crossref] [PubMed]
- Tornovsky-Babeay S, Dadon D, et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in β cells. Cell Metab 2014;19:109-21. [Crossref] [PubMed]
- Mah LJ, El-Osta A, Karagiannis TC. GammaH2AX as a molecular marker of aging and disease. Epigenetics 2010;5:129-36. [Crossref] [PubMed]
Cite this article as: Lippi G, Tarperi C, Danese E, Montagnana M, Festa L, Benati M, Salvagno GL, Bonaguri C, Bacchi E, Donà S, Roggenbuck D, Moghetti P, Schena F. Middle-distance running and DNA damage in diabetics. J Lab Precis Med 2018;3:18.


