
Prognostic value of systemic immune-inflammation index (SII) in cancers: a systematic review and meta-analysis
Introduction
The inflammatory response in tumor microenvironment plays an essential role in cancer development and progression (1,2). Various measurable blood parameters can mirror the systemic inflammatory response which includes but not limited to C-reactive protein, red blood cell distribution width, hypoalbuminemia, cytokines such as interleukin-6 and tumor necrosis factor-α, and leucocyte and their subtype counts (3-5). Cancer-associated inflammation is regarded as a hallmark characteristic of tumor development. Therefore, inflammatory biochemical markers have been incorporated in the prognostic scoring systems of several types of cancer (6,7).
It has increasingly been acknowledged that these inflammatory cells are involved in the production of inflammatory mediators and cytokines that can affect angiogenesis, growth, invasion, and metastasis of the cancers (8). Correspondingly, serum neutrophils, lymphocytes and platelets have been known to predict prognosis and response to the treatment in different malignancies (9). In 2014, Hu et al. reported a novel inflammatory index, named as the systemic immune-inflammation index (SII) based on counts of lymphocyte (L), neutrophil (N), and platelet (P), and it was calculated as follows: SII = P × N/L (10). SII was proven to be a strong independent predictive factor for recurrence and prognosis in patients with hepatocellular carcinoma (HCC). It was concluded that the high recurrence rate in patients with high SII scores might be related to increased release of circulating tumor cells (CTCs) from tumor sites.
Recently, SII was recently explored as a prognostic marker in several other malignancies including renal cell carcinoma and small cell lung cancer (SCLC) (10-12). However, the prognostic value of SII for survival in cancer patients remains conflicting. Therefore, our study focuses on obtaining a combined and unified result based on the several past independent studies which assessed the prognostic value of SII in clinical outcomes in various solid tumors. We hypothesized that SII can be a readily available, objective and inexpensive prognostic index which could be used in the stratification of cancer patients in daily oncological practice.
Methods
Literature source and search
The full search strategy and method are described and attached in the supplementary material (available online). We searched PubMed, Cochrane Library, American Society of Clinical Oncology (ASCO), and European Society for Medical Oncology (ESMO) databases on or up to April 20, 2017. All references incorporated into the literature were manually retrieved. The following terms were used: (I) “systemic immune-inflammation index” in conjunction with (II) “cancer, or tumor, or neoplasm, or carcinoma” and (III) “prognosis, or outcome, or survival, or mortality, or recurrence, or progression, or metastasis.”
Study selection and data extraction
Titles and abstracts were screened and assessed to meet the following inclusion criteria: (I) studies reporting the prognostic impact of the peripheral blood SII in patients with solid tumors; and (II) reporting of a hazard ratio (HR) and 95% confidence interval (CI) for overall survival (OS). For secondary analysis, studies providing a HR for cancer-specific survival (CSS), progression-free survival (PFS), time to recurrence (TTR) were included as well.
Literature was screened by following the inclusion criteria, and data was extracted and crosschecked by two independent reviewers. The following details were extracted: first author’s name, type of the publication (abstract, full text), year of publication, number of patients included in the analysis, primary cancer site, cancer stage, type of data collection (prospective, retrospective). SII cutoff and HRs were extracted from multivariable analyses where available. Otherwise, HRs from univariate analyses were included in the analysis. The methodology of each study was appraised using a quality score ranged from 0 to 9 based on the Newcastle-Ottawa assessment scale (NOS) (13). The quality score assesses the three major aspects of studies: selection, comparability, and the outcome of study groups. A higher quality score indicated good design with the availability of adequate information in the study.
Statistical analyses
Extracted data were integrated into meta-analysis using Rev Man 5.1 analysis software. Estimation of HRs was weighted and pooled using the generic inverse-variance and random-effect model (14). All studies were included in the analysis, and differences between the subgroups were assessed using methods described (14). The heterogeneity assumption of the model was assessed by Cochrane Q test and inverse-variance method, where a P value <0.10 and I2≥50% indicated a significant heterogeneity across studies. Due to the existence of considerable heterogeneity in the studies, a meta-regression analysis was performed to identify heterogeneous factor (STATA 12). We assessed the publication bias of extracted data using the Begg’s test and Begg’s funnel plots (STATA 12). A sensitivity analysis was performed to examine the robustness of our overall findings to potentially uncertain or influential inputs. All statistical analyses were two-sided, and statistical significance was defined as P<0.05.
Results
Included studies
We identified a total of 170 records from the primary literature search, of which 13 studies with a total of 4,104 patients from December 2014 to January 2017 met the inclusion criteria (Figure 1) (10-12,15-24) . Since the Hu et al.’s paper included two independent cohorts studies, a total 14 cohorts were included in the final analysis (10) Characteristics of comprised studies are shown in Table 1. The utility of SII was reported after multivariate analysis in all but one of the studies. Twelve studies shows the quality score in the range of 6–8 indicating sufficient quality for our meta-analysis, and for Yu et al.’s study could not be evaluated because of unavailability of adequate clinical information.
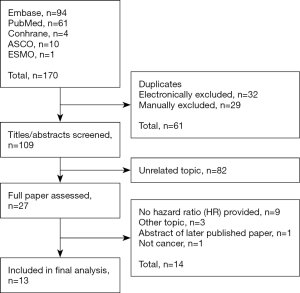
Table 1
| Study | Study type | Country | Ethnicity | Cancer type | Sample size | outcome | Cut off value (×109) | NOS |
|---|---|---|---|---|---|---|---|---|
| Hu et al., 2014 | SRCS | China | Asian | Hepatocellular carcinoma | 133 | OS,TTR | 330 | 6 |
| Hu et al., 2014 | SPCS | China | Asian | Hepatocellular carcinoma | 123 | OS,TTR | 330 | 7 |
| Hong et al., 2015 | SRCS | China | Asian | Small cell lung cancer | 919 | OS | 1,600 | 6 |
| Yang et al., 2015 | SRCS | China | Asian | Hepatocellular carcinoma | 189 | OS | 300 | 6 |
| Gardini et al., 2016 | SRCS | Italy | Caucasian | Hepatocellular carcinoma | 56 | OS | 360 | 6 |
| Geng et al., 2016 | SRCS | China | Asian | ESCC | 916 | OS | 307 | 6 |
| Huang et al., 2016 | SRCS | China | Asian | Gastric cancer | 455 | OS | 572 | 6 |
| Lollis et al., 2016 | SRCS | Italy | Caucasian | Renal cell cancer | 335 | PFS,OS | 730 | 6 |
| Lollis et al., 2016 | SRCS | Italy | Caucasian | Prostate cancer | 230 | OS | 535 | 6 |
| Passardi et al., 2016 | MPRS | Italy | Caucasian | Colorectal cancer | 289 | PFS,OS | 730 | 8 |
| Wang et al., 2016 | SRCS | China | Asian | Hepatocellular carcinoma | 163 | TTR | 330 | 6 |
| Yu et al., 2016* | SRCS | China | Asian | Acral melanoma | 113 | OS,TTR | 615 | NA |
| Feng et al., 2017 | SRCS | China | Asian | ESCC | 298 | CSS | 410 | 6 |
| Gao et al., 2017 | SRCS | China | Asian | Hepatocellular carcinoma | 183 | OS TTR | 330 | 6 |
*, the type of publication is conference abstract. SRCS, single center retrospective cohort study; SPCS, single center prospective cohort study; MPRS, multicenter prospective randomized study; ESCC, esophageal squamous cell carcinoma; OS, overall survival; PFS, progression-free survival; TTR, time to recurrence; CSS, cancer-specific survival; NA, not available; NOS, Newcastle-Ottawa scale.
Meta-analysis results
As shown in Figure 2, The combined results indicated that elevated SII was associated with poor OS (HR =1.80; 95% CI: 1.43–2.28), poor CSS (HR =1.44; 95% CI: 1.04–1.99), TTR (HR =1.91; 95% CI: 1.53–2.43) and poor PFS (HR =1.18; 95% CI: 0.55–2.52). Except the endpoint of TTR (I2=0%, P=0.78), the rest of meta-analysis demonstrated significant heterogeneity.
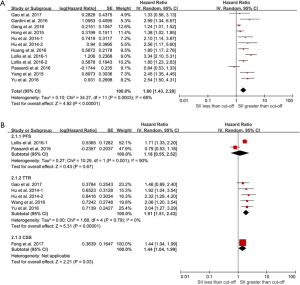
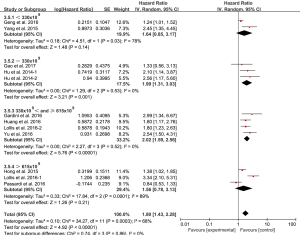
Because of the presence of significant heterogeneity, a comprehensive subgroup analysis was conducted. The combined effect of SII on OS among cancer types is shown in Figure 3A.The significant prognostic effect of SII was highest in renal cell cancer (HR =3.34; 95% CI: 2.10–5.31), followed by acral melanoma (HR =2.54; 95% CI: 1.50–4.31), HCC (HR =2.25; 95% CI: 1.64–3.09), prostate cancer (HR =1.80; 95% CI: 1.23–2.63), gastric cancer (HR =1.80; 95% CI: 1.17–2.76), SCLC (HR =1.38; 95% CI: 1.02–1.85), esophageal squamous cell carcinoma (ESCC) (HR =1.24; 95% CI: 1.01–1.52), but was not shown in colorectal cancer (HR =0.84; 95% CI: 0.53–1.33). Moreover, a subgroup analysis (stratified by sample size, cutoff value, ethnicity, and study type) for OS, demonstrated statistically significant HR in all defined subgroups (Figure 3B,C,D). However, in the prospective analysis cut-off <330×109, and cut-off >615×109 subgroups, the negative association was found between the SII and OS (Figure 3C, Figure 4).

Sensitivity analyses
Sensitivity analyses were conducted for the meta-analysis of OS by excluding one study at a time, and statistical results were compared. We found that heterogeneity changed between 58–71% and P value remained stable (all P<0.0001), which suggest that our overall findings were robust without potentially uncertain or influential inputs.
Publication bias and meta-regression
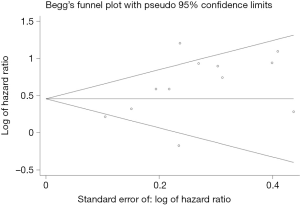
Publication biases were assessed by Begg’s test and Begg’s funnel plot for meta-analysis of OS (Figure 5). We did not find any publication biases (P=0.17). In order to identify the heterogeneity factor between main available factors (sample size, cut off value, cancer type, ethnicity and study type), meta-regression analysis was conducted. The result showed that any these main factors did not function as heterogeneity factor (data not shown).
Discussion
Recently published studies have suggested that an elevated SII is associated with poor survival in subjects with cancer. Herein, we undertook a meta-analysis of 13 studies (14 cohorts) comprising 4,012 patients to assess the prognostic utility of pretreatment SII in patients with solid tumors. The combined results indicated that elevated SII significantly predicted poor OS, poor CSS, poor TRR and poor PFS in this patient population. We found a high SII is consistently associated with survival (HR =1.80) among different subgroups.
Inflammation has been reported to be a contributing factor in the development of many cancers and is now considered as a hallmark characteristic in all tumors (25). In recent years, the neutrophil-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratios (PLR) have been suggested as simple indices of systemic inflammatory response and a prognostic indication in different kinds of cancers (26,27).The SII was constructed based on lymphocyte, neutrophil, and platelet counts. Initially, SII was first shown to indicate the host inflammatory and immune status in resected HCC patients. In this study, SII was found to be one of the strongest predictive ability among other indices (NLR, PLR, and other parameters in predicting survival and recurrence), and possibly associated with CTCs levels (10). Later, Hong et al. and Yang et al. demonstrated that SII could also help in the prediction of poor prognosis in SCLC and HBV-related HCC, respectively (12,15). Many other studies were published later which showed the prognostic ability of SII in different kinds of cancers (11,18-21,24). Recently dynamic changes in SII were shown to be useful to predict the prognosis of patients with resected HCC (16). However, the study by Passardi et al. showed an insignificant association between SII and OS in metastatic colorectal cancer (17).
In our study, the results of the primary meta-analysis and most of the subgroup analysis showed that SII is indeed a robust predictive index for OS and TRR, which is consistent with previous reports (10,12,18,20). The prognostic values of SII in cancers can be explained by four types of cells (10). The first kind of cells are the CTCs which play a crucial role in the initiation of recurrence and metastases of cancer after surgery (28). The second type of cells are the neutrophils which secretes circulating growth factors promoting cancer cells adhesion and seeding in distant organ sites, as well as assisting cancer cells in evading immune surveillance (25,29). The third type of cells are the lymphocytes which play a vital role in tumor defense by inhibiting tumor cell proliferation and migration and inducing cytotoxic cell death (26). The fourth type of cells are the platelets which have emerged as a vital player in the systemic and local response to the tumor growth (27). Moreover, recent evidence indicates that platelets could facilitate tumor cell survival and metastasis (29,30).
We also did not find statistical significance between the prognostic value of SII and survival in colorectal cancer subgroup, cut-off <330×109 subgroup, cut-off of >615×109 subgroup, and prospective study subgroup. Study by Passardi et al. included most of the above insignificant subgroups, except for cut-off of <330×109 subgroup. Moreover, limited number of other studies had the four subgroup analysis. Thus, the presented insignificant results seem not be reliable, and these results warrant further studies to validate.
Our study has some limitations which are worth to point out. One of the limitation of our meta-analysis is a small number of published studies with the specific subgroup which could affect the interpretation. Furthermore, most of these studies were retrospective, and the cutoff values of SII were not consistent. We also found another meta-analysis on prognostication value of SII, but this meta-analysis did not include the combined value of SII in TTR which is the end point in many studies (31). Our analysis is more comprehensive with four subgroups of SII cutoffs.
In conclusion, we performed a comprehensive meta-analysis revealing that the elevated SII is an unfavorable predictor of prognosis in patients with cancers. SII can be used a potential prognostic indicator, and further large-scale prospective studies are warranted to investigate the efficacy of SII in clinical decision making in patients with cancers.
Acknowledgments
Funding: This study was supported by Health and Family Planning Commission of Sichuan Province universal application project (No.17PJ516).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.03.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- O'Callaghan DS, O'Donnell D, O'Connell F, et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol 2010;5:2024-36. [Crossref] [PubMed]
- Roxburgh CS, Mcmillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149-63. [Crossref] [PubMed]
- Goyal H, Hu ZD. Prognostic value of red blood cell distribution width in hepatocellular carcinoma. Ann Transl Med 2017;5:271. [Crossref] [PubMed]
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017;23:4879-91. [Crossref] [PubMed]
- Mcmillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534-40. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget 2017;8:16027-35. [PubMed]
- Hu H, Yao X, Xie X, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol 2017;35:261-70. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Geng Y, Shao Y, Zhu D, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Sci Rep 2016;6:39482. [Crossref] [PubMed]
- Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:297-304. [Crossref] [PubMed]
- Wells GA, Shea BJ, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Appl Eng Agric 2014;18:727-34.
- Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. In: The Cochrane Collaboration. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie 2011;2011:S38.
- Yang Z, Zhang J, Lu Y, et al. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget 2015;6:43090-8. [Crossref] [PubMed]
- Wang B, Tian L, Gao XH, et al. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med 2016;54:1963-9. [Crossref] [PubMed]
- Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 2016;7:33210-9. [Crossref] [PubMed]
- Lolli C, Basso U, Derosa L, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget 2016;7:54564-71. [Crossref] [PubMed]
- Lolli C, Caffo O, Scarpi E, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front Pharmacol 2016;7:376. [Crossref] [PubMed]
- Huang L, Liu S, Lei Y, et al. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget 2016;7:44185-93. [PubMed]
- Casadei Gardini A, Scarpi E, Faloppi L, et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget 2016;7:67142-9. [PubMed]
- Gao XH, Tian L, Wu J, et al. Circulating CD14+ HLA DR−/low myeloid derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res 2017;47:1061-71. [Crossref] [PubMed]
- Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore) 2017;96:e5886 [Crossref] [PubMed]
- Yu J, Li SM, Kong Y, et al. Association of immune-inflammation index with outcome of high-risk acral melanoma patients treated with adjuvant high-dose interferon. J Clin Oncol 2016;34:e21070
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [Crossref] [PubMed]
- Templeton AJ, Mcnamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124 [Crossref] [PubMed]
- Zhou X, Du Y, Huang Z, et al. Prognostic Value of PLR in Various Cancers: A Meta-Analysis. PLoS One 2014;9:e101119 [Crossref] [PubMed]
- Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 2012;13:688-95. [Crossref] [PubMed]
- Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013;24:130-7. [Crossref] [PubMed]
- Labelle M, Begum S, Hynes RO. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell 2011;20:576-90. [Crossref] [PubMed]
- Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017;8:75381-8. [Crossref] [PubMed]
Cite this article as: Tang JN, Goyal H, Yu S, Luo H. Prognostic value of systemic immune-inflammation index (SII) in cancers: a systematic review and meta-analysis. J Lab Precis Med 2018;3:29.


