
Critical appraisal to using relative or absolute cardiac troponins change for diagnosing acute myocardial infarction
Both cardiac troponins T (cTnT) and I (cTnI) are universally considered the reference biomarkers for diagnosing acute myocardial infarction (AMI) (1). Irrespective of the guidelines or recommendations used for diagnosing AMI, the conventional approach entails a baseline measurement eventually followed (i.e., non-diagnostic values at the first measurement or suggestive electrocardiogram changes) by serial sampling at different times points, which are aimed to detect suggestive changes of cardiac troponins (cTn) reflective of an acute ischemic event (2,3). Two different strategies have been proposed for interpreting results of serial cTn testing, the former entailing an absolute variation of concentration from the baseline value (i.e., expressed in cTn concentration, ng/L), and the latter based on a relative change from the baseline value (i.e., expressed in percentage increase, %). The most commonly used cutoffs of cTn variation used for serial sampling encompass an absolute delta comprised between 5 and 10 ng/L or a ~20% variation, since these thresholds actually reflect the within-intra-individual biological variation of cTn in emergency department patients (4).
Though many studies have been published on the diagnostic efficiency of both strategies and currently there is general consensus that the use of the absolute variation may be more clinically useful for both diagnosing and ruling out AMI (5,6), absolute changes of cTn concentration may occur for non-biological causes, i.e., due to preanalytical or analytical issues (7). The latter aspect is especially significant. Although the new generation of high-sensitivity (HS) cTn immunoassays is characterized by considerably magnified analytical performance (including a lower analytical imprecision) (8,9), an improved analytical variation may impact the efficiency of diagnostic algorithms based on absolute cTn variation during serial sampling.
A pragmatic representation of this issue is given in Figure 1A, showing the confidence interval (CI) limits of Roche HS cTnT values based on a previously estimated analytical imprecision (intra-assay coefficient of variation; CV) of 8.4% (10), which is then applied to either an absolute increase of 5 ng/L (as suggested by Marjot et al.) (11) or the conventional 20% increase from the baseline cTnT value. The baseline value (straight black line) would never be included within the CI limits drawn according to the level of analytical imprecision of the method when the diagnostic threshold is based on the 20% increase of cTnT from baseline (dotted blue lines). On the other hand, for cTnT concentrations >50 ng/L, the baseline values would be included within the CI limits drawn according to the level of analytical imprecision of the method when the diagnostic threshold is based on a 5 ng/L increase of cTnT from baseline (dotted blue lines). Practically, this means that the window of analytical imprecision of the cTnT immunoassay may virtually impair the diagnostic performance of the absolute increase after a certain concentration (i.e., 50 ng/L for cTnT).
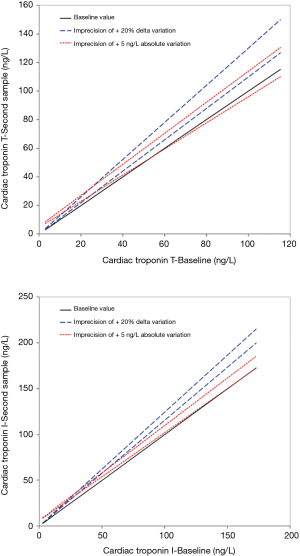
The absolute variation may exhibit better diagnostic performance when using HS troponin immunoassays with lower analytical imprecision. Figure 1B shows the CI limits of Abbott HS cTnI values based on a previously estimated analytical imprecision (mean intra-assay CV) of 3.7% (12), which is then applied to either an absolute increase of 6 ng/L (as suggested by Neumann et al.) (13) or the conventional 20% increase from the baseline cTnI value. Like cTnT, the baseline value (straight black line) would never be included within the CI limits drawn according to the level of analytical imprecision of the method when the diagnostic threshold is based on the 20% increase of cTnI from baseline (dotted blue lines). However, for cTnI concentrations >150 ng/L the baseline values would be included within the CI limits drawn according to the level of analytical imprecision of the method when the diagnostic threshold is based on a 6 ng/L increase of cTnI from baseline (dotted blue lines). This means, again, that the window of analytical imprecision of the cTnI immunoassay may virtually impair the diagnostic performance of the absolute increase after a certain concentration (i.e., 150 ng/L for Abbott cTnI).
This straightforward concept can hence be easily deployed to all the commercially available cTnI and cTnT HS immunoassays, by knowing in advance the analytical imprecision and the optimal thresholds for both relative and absolute variations of the method. Although it can be argued that the threshold over which the CI limits of imprecision will include the baseline cTn values are relatively distant from the 99th upper reference limit (URL; 13 ng/L for cTnT and 27 ng/L for cTnI, respectively), cTn concentrations as high as 6.5-fold the URL are frequently observed in patients admitted to the emergency department with non-ischemic chest pain. These patients, who are finally diagnosed with myocardial injuries different from AMI, regularly undergo serial cTn monitoring for ruling out acute myocardial ischemia (11,14). In these conditions, values as high as 85 ng/L for cTnT and 176 for cTnI can be observed, thus exceeding, in both cases, the thresholds after which the imprecision of the cTn immunoassays would make it unadvisable using the absolute variation (i.e., 50 and 150 ng/L, respectively).
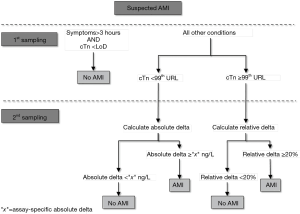
Taken together, the data emerged from our analysis seemingly shows that the relative change may outperform (and may be also considered a safer approach) the absolute variation in patients presenting with intermediate or high cTn values (Figure 1). On the other hand, this strategy may be less efficient for diagnosing AMI in patients presenting with low cTn values or when using narrow serial testing (i.e., 1- or even 2-hour protocols), so that the absolute change may generally appear more clinically and analytically reliable in these conditions (5). Pragmatic working solutions may thus entail the use of absolute cTn increases when the concentration is lower than the 99th URL, whilst the 20% variation may then be more analytically robust and reliable for cTn values above such limit, as shown in Figure 2. This may henceforth lead the proposal of a revised diagnostic algorithm for AMI, which will need to be tested in future studies, combining symptoms onset, analytical characteristics of cTn immunoassay (namely the limit of detection and the URL), as well as the absolute and relative cTn variation (see Figure 3).

Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.04.09). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Fabian Sanchis-Gomar serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from December 2016 to November 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cervellin G, Lippi G. Of MIs and men--a historical perspective on the diagnostics of acute myocardial infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref] [PubMed]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
- Lindahl B, Venge P. Early rule out of acute myocardial infarction. J Lab Precis Med 2017;2:53. [Crossref]
- Simpson AJ, Potter JM, Koerbin G, et al. Use of observed within-person variation of cardiac troponin in emergency department patients for determination of biological variation and percentage and absolute reference change values. Clin Chem 2014;60:848-54. [Crossref] [PubMed]
- Herman DS, Kavsak PA, Greene DN. Variability and Error in Cardiac Troponin Testing: An ACLPS Critical Review. Am J Clin Pathol 2017;148:281-95. [Crossref] [PubMed]
- van Doorn WP, Vroemen WH, de Boer D, et al. Clinical laboratory practice recommendations for high-sensitivity cardiac troponin testing. J Lab Precis Med 2018;3:30. [Crossref]
- Sanchis-Gomar F, Lippi G. Physical activity - an important preanalytical variable. Biochem Med (Zagreb) 2014;24:68-79. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Aloe R, Bonfanti L, Salvagno GL, Cervellin G. High-sensitivity cardiac troponin I immunoassay reduces the chance of patient misclassification in the emergency department. J Lab Precis Med 2017;2:93. [Crossref]
- van der Linden N, Streng AS, Wodzig WK, et al. Better, higher, lower, faster: increasingly rapid clinical decision making using high-sensitivity cardiac troponin assays. J Lab Precis Med 2017;1:14. [Crossref]
- Koerbin G, Tate JR, Hickman PE. Analytical characteristics of the Roche highly sensitive troponin T assay and its application to a cardio-healthy population. Ann Clin Biochem 2010;47:524-8. [Crossref] [PubMed]
- Marjot J, Kaier TE, Henderson K, et al. A single centre prospective cohort study addressing the effect of a rule-in/rule-out troponin algorithm on routine clinical practice. Eur Heart J Acute Cardiovasc Care 2017;2048872617746850 [Epub ahead of print]. [PubMed]
- Lee K, Lee SY, Choi JO, et al. The distribution of Abbott high-sensitivity troponin I levels in Korean patients with chest pain. Ann Clin Lab Sci 2015;45:152-7. [PubMed]
- Neumann JT, Sorensen NA, Ojeda F, et al. Early diagnosis of acute myocardial infarction using high-sensitivity troponin I. PLoS One 2017;12:e0174288 [Crossref] [PubMed]
- Tecson KM, Arnold W, Barrett T, et al. Interpretation of positive troponin results among patients with and without myocardial infarction. Proc (Bayl Univ Med Cent) 2017;30:11-5. [Crossref] [PubMed]
Cite this article as: Lippi G, Cervellin G, Sanchis-Gomar F. Critical appraisal to using relative or absolute cardiac troponins change for diagnosing acute myocardial infarction. J Lab Precis Med 2018;3:43.


