
Phosphorylated histone 2AX foci determination in capillary blood mononuclear cells
Introduction
Histone 2AX (H2AX) phosphorylation at serine 139 is an early event in the cellular repair of DNA double-strand breaks (DSB) representing one of the most severe forms of DNA damage (1). Phosphorylated H2AX protein (γH2AX) was established as a useful biomarker for genotoxicity testing, anti-cancer drug development, and assessment of individual chemo- and radio-responsiveness (2-4). Peripheral blood mononuclear cells (PBMCs) isolated from venous blood are an ideal substrate for efficient γH2AX foci detection (5,6).
The most sensitive method to quantify γH2AX foci is the indirect immunofluorescence assay (IFA) employing staining with anti-γH2AX antibodies (7,8). However, manual interpretation of γH2AX foci in IFA is a subjective and time-consuming approach. Recently, automated fluorescence microscopy in combination with novel pattern recognition software has enabled the standardized detection of γH2AX foci in cell nuclei (8). Thus, γH2AX foci counting and intensity analysis per cell as well as morphological analysis of cell nuclei and foci can be achieved automatically (3,5,8-10).
Other commonly used techniques like western blot analysis or flow cytometry are useful methods for γH2AX assessment, but less sensitive and not able to analyze γH2AX foci on a single cell and foci level, respectively (8).
Frequently, human PBMCs have been used as substrate for the γH2AX foci analysis in the context of DNA damage assessment. In general, approx. 3–5 mL blood was drawn by venipuncture and a sufficient number of cells isolated by density gradient centrifugation. However, to achieve a high confidence level, at least 100 cells per sample should be analyzed (11).
Although venipuncture is a minimal invasive method, there is a risk for paravenous or complete vein penetration with formation of hematoma and scars. Furthermore, repeated venipuncture is a stressing procedure for children and older people. Altogether, this creates the need for an even less invasive method of blood sampling for γH2AX foci analysis. Recently, Heylmann et al. published a new approach to detect γH2AX foci by using a drop of blood for a full blood smear (12,13). However, the density of capillary blood mononuclear cells (CBMC) by this method is not sufficient for automated evaluation. Furthermore, Turner et al. evaluated the RABIT system as high throughput γH2AX analyzing machine (14). Thereby, γH2AX total fluorescence in CBMCs was investigated, resulting in a less sensitive mean value over all CBMCs.
Thus, the aim of this study was to find a new method for the isolation of a sufficient amount of CBMCs from one drop of capillary blood and compare it with the current technique employing a larger volume of venous blood.
Methods
Ten healthy volunteers were recruited which gave written informed consent [female 6, male 4; median age: 32 years; interquartile range (IQR): 8 years]. The study was approved by the local ethics committee and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Blood donors gave written informed consent.
PBMC isolation
Mononuclear cells were isolated from venous blood taken by venipuncture using a commercial lymphocyte separation kit (Medipan GmbH, Dahlewitz/Berlin, Germany).
CBMC isolation
Capillary finger stick blood was obtained using a lancet and capillary tube K2-EDTA with collector unit. Subsequently, a density gradient was employed in 1.5 mL centrifuge tubes to separate the CBMCs from other blood cells. Obtained CBMCs were washed in PBS to remove remaining density gradient medium.
Cell exposure to etoposide
Immediately after isolation, PBMC and CBMC were suspended in commercial ready to use the Roswell Park Memorial Institute (RPMI) 1,640 medium and exposed to differing levels of etoposide (0–100 µM) for one hour at 37 °C and 5% CO2 (3). Etoposide is a well characterized and widely used cytostatic drug. It is a topoisomerase II poison which blocks DNA ligation resulting in DBS (12).
After incubation, etoposide was removed by centrifugation and washing with PBS. Cells were seeded on glass slide and fixed as described elsewhere (8).
Indirect IFA for the detection of γH2AX
According to the instruction manual (Medipan GmbH), PBMCs and CBMCs were permeabilized and stained with an anti-γH2AX mouse monoclonal primary antibody for one hour at room temperature as described earlier (8). This was followed by washing and incubation with a specific anti-mouse secondary antibody labeled with fluorescein isothiocyanate (FITC) for one hour at room temperature. After incubation of the secondary antibody, unbound components were removed by washing and the wells were covered with a 4’, 6-diamidino-2-phenylindole (DAPI) containing mounting medium.
Automated cell and foci analysis
Image acquisition and interpretation was performed with the AKLIDES Cell Damage system (Medipan) as described in detail previously (8). Briefly, DAPI stained cell nuclei were used for autofocusing and analysis of morphological characteristics (shape, size, intensity). Only DAPI stained nuclei with an almost perfectly circular shape (convexity of 0.85–1.0) were automatically selected for further γH2AX analysis. The AKLIDES Cell Damage software uses mathematical algorithms for digital cell separation and z-stack foci acquisition as well as foci analysis regarding their number, intensity and morphological characteristics.
For validation of IFA results and minimizing preanalytical variability, polymethylmethacrylate (PMMA) microparticles (PolyAn, Berlin, Germany) functionalized with a carboxylated surface were used as staining control. These particles were covalently linked with γH2AX peptide. Subsequently, they were immobilized on a separate well of the microscopic glass slide and used for IFA analysis (Figure 1A). In order to guarantee a high level of reproducibility regarding IFA analysis, the homogenous γH2AX PMMA microbead density of different lots was controlled by anti-γH2AX and labeled secondary antibodies. The analysis of the median fluorescence intensity (MFI) for comparison was based on the detection of the fluorescent signal of 100 beads each of four sites per well on the microscopic glass slides. Thus, the MFI measurements of different slides after running the IFA allowed comparing the efficiency of assay performance regarding assay conditions and binding of specific antibodies.
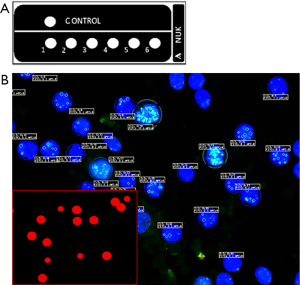
The AKLIDES Cell Damage software analyzes the MFI being a result of the bound anti-γH2AX and secondary antibodies as described above. Ligand values ranging from 37,280 AU to 71,375 AU were considered valid for further γH2AX analysis of specimens as mentioned before and shown in Figure 1B. Values out of range were scored not valid and respective slides were not investigated further.
Statistics
Statistical analysis was done as described in detail previously (3,5,8).
Results
All obtained data showed controlled, valid results using γH2AX coated microparticles, ranging in the appropriate value as mentioned before (data not shown).
Comparison of PBMCs and CBMCs obtained by venipuncture and capillary puncture
The isolated PBMCs and CBMCs were compared in terms of their number and nuclear characteristics. Taking 50 µL of peripheral and capillary blood each, there was no significantly different cell number of obtained PBMCs and CBMCs. Thus, the amount of 50 µL capillary blood was sufficient for the isolation of a number of at least 500 CBMCs that could be used for the automated IFA interpretation of duplicates. The scanning and evaluation time of minimum 100 PBMCs or CBMCs in one well was similar and lasted 4–5 minutes.
Both isolation strategies resulted in the isolation of PBMCs and CBMCs with a median nucleus diameter of 7.2 to 7.4 µm with no significant difference (Figure 2A).
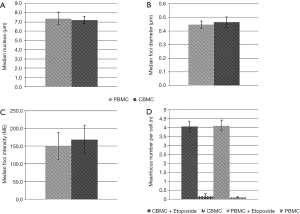
Comparison of etoposide-induced foci characteristics
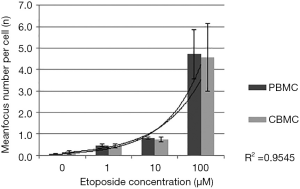
DSBs were induced by treatment with 100 µM etoposide in PBMCs and CBMCs obtained via venous and capillary puncture. The median foci diameter and median foci intensity showed no significant differences in both techniques (PBMCs: 0.45 µm, CBMCs: 0.46 µm; foci intensity as MFI: 150.4–169.2, respectively) (Figure 2B,C). Further, we compared DSB formation after exposure at baseline and to 100 µM etoposide in PBMCs and CBMCs. There were no significant differences in DSB formation (P>0.05, respectively (Figure 2D). Additionally, samples were treated with different etoposide concentrations (1, 10, 100 µM) and the mean γH2AX foci numbers were determined by counting foci in at least 100 nuclei per specimen (Figure 3). Foci numbers ranged from 0.074 at baseline (0 µM etoposide) to 4.720 foci per cell after exposure to 100 µM etoposide. There was an exponential relationship of the concentration of etoposide with the number of induced γH2AX foci in PBMCs and CBMCs (coefficient of determination = R2=0.96) (Figure 3).
Discussion
Recent studies have underlined the usefulness of capillary puncture to obtain blood specimens in different diagnostic fields and experimental settings. Especially for pediatric and multimorbid patients capillary puncture is less cumbersome and stressful in contrast to venipuncture. However, Schalk et al. showed in a multi-cohort study a significant elevation of white blood cell numbers in finger stick (capillary) versus venous blood (15). In contrast, a less extensive cohort study of Podgorski et al. demonstrated no significant differences of blood cell counts in capillary and venous blood (16). This finding is consistent with our results. Further, we determined no significant differences of nuclei and γH2AX foci characteristics. By applying automated IFA interpretation with the AKLIDES system, a controlled analysis with synthetic microparticles minimizes the variability of γH2AX foci numbers.
As a fact, biochemical parameters may demonstrate different levels in capillary and venous blood samples (17-19). In contrast, we found no significant differences in the formation and characteristics of etoposide-induced DSBs determined by automated γH2AX foci analysis in this in vitro study.
Conclusions
In sum, our results demonstrate that CBMCs can be used instead of PBMCs for the assessment of DNA damage by γH2AX foci analysis. This may facilitate the introduction of γH2AX as a biomarker into routine diagnostics. The use of capillary puncture as the most minimal invasive and less complicated blood taking method can enable easier sample procurement for more comprehensive DNA damage assessment studies.
Of note, therapy-induced or preexisting bone marrow failure may result in low CBMC counts which could limit the applicability of the method in daily routine. Additionally, it needs to be investigated in further studies whether the induction of DSBs in vivo can be detected equally in PBMCs and CBMCs and whether the number of affected CBMCs with DSBs is sufficient for an appropriate analysis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “DNA Damage Assessment for Precision Medicine”. The article has undergone external peer review.
Conflicts of Interest: The series “DNA Damage Assessment for Precision Medicine” was commissioned by the editorial office without any funding or sponsorship. Dirk Roggenbuck served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from August 2017 to July 2019. D Roggenbuck is a shareholder and employee of GA Generic Assays GmbH and Medipan GmbH. M Sowa receives a grant from GA Generic Assays GmbH. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committee (#38/14) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burma S, Chen BP, Murphy M, et al. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001;276:42462-7. [Crossref] [PubMed]
- Paull TT, Rogakou EP, Yamazaki V, et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000;10:886-95. [Crossref] [PubMed]
- Reddig A, Lorenz S, Hiemann R, et al. Assessment of modulated Cytostatic Drug Resistance by Automated γH2AX Analysis. Cytometry A 2015;87:724-32. [Crossref] [PubMed]
- Siddiqui MS, François M, Fenech MF, et al. Persistant γH2AX: A promising molecular marker of DNA damage and Aging. Mutat Res Rev Mutat Res 2015;766:1-9. [Crossref] [PubMed]
- Reddig A, Fatahi M, Friebe B, et al. Analysis of DNA Double-Strand Breaks and Cytotoxicity after 7 Tesla Magnetic Resonance Imaging of Isolated Human Lymphocytes. PLoS One 2015;10:e0132702 [Crossref] [PubMed]
- Redon CE, Nakamura AJ, Sordet O, et al. γ-H2AX Detection in Peripheral Blood Lymphocytes, Splenocytes, Bone Marrow, Xenografts, and Skin. Didenko V V (Hrsg). Methods Mol Biol 2011;682:249-70.
- Rothkamm K, Horn S. γ-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita 2009;45:265-71. [PubMed]
- Willitzki A, Lorenz S, Hiemann R, et al. Fully automated analysis of chemically induced γH2AX foci in human peripheral blood mononuclear cells by indirect immunofluorescence. Cytometry A 2013;83:1017-26. [Crossref] [PubMed]
- Runge R, Hiemann R, Wendisch M, et al. Fully automated interpretation of ionizing radiation-induced γH2AX foci by the novel pattern recognition system AKLIDES®. Int J Radiat Biol 2012;88:439-47. [Crossref] [PubMed]
- Runge R, Arlt J, Oehme L, et al. Comparison of clonogenic cell survival and DNA damage induced by 188Re and X-rays in rat thyroid cells. Nuklearmedizin 2017;56:47-54. [Crossref] [PubMed]
- Redon CE, Nakamura AJ, Sordet O, et al. γ-H2AX Detection in Peripheral Blood Lymphocytes, Splenocytes, Bone Marrow, Xenografts, and Skin. Methods Mol Biol 2011;682:249-70. [Crossref] [PubMed]
- Pommier Y, Leo E, Zhang H, et al. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol 2010;17:421-33. [Crossref] [PubMed]
- Heylmann D, Kaina B. The γH2AX DNA damage assay from a drop of blood. Sci Rep 2016;6:22682. [Crossref] [PubMed]
- Turner HC, Brenner DJ, Chen Y, et al. Adapting the γ-H2AX assay for automated processing in human lymphocytes. 1. Technological aspects. Radiat Res 2011;175:282-90. [Crossref] [PubMed]
- Schalk E, Heim MU, Koenigsmann M, et al. Use of capillary count parameters in adults. Vox Sang 2007;93:348-53. [PubMed]
- Podgorski T, Bartkowiak U, Pawlak M. Comparison of hematological parameters of venous and capillary blood in athletes. Trends in Sport Sciences 2014;21:39-45.
- Jones AW, Jönsson KA, Jorfeldt L. Differences between capillary and venous blood-alcohol concentrations as a function of time after drinking, with emphasis on sampling variations in left vs right arm. Clin Chem 1989;35:400-4. [PubMed]
- Boyd R, Leigh B, Stuart P. Capillary versus venous bedside blood glucose estimations. Emerg Med J 2005;22:177-9. [Crossref] [PubMed]
- Kupke IR, Kather B, Zeugner S. On the composition of capillary and venous blood serum. Clin Chim Acta 1981;112:177-85. [Crossref] [PubMed]
Cite this article as: Sowa M, Reddig A, Schierack P, Reinhold D, Roggenbuck D. Phosphorylated histone 2AX foci determination in capillary blood mononuclear cells. J Lab Precis Med 2018;3:45.


