
Quantification of DNA double-strand breaks in peripheral blood mononuclear cells from healthy donors exposed to bendamustine by an automated γH2AX assay—an exploratory study
Introduction
DNA double strand breaks (DSB) constitute a major and, if unrepaired, fatal form of DNA damage which usually leads to apoptotic cell death. They seem to occur spontaneously at a very low rate, and have otherwise been attributed to cell stress of any kind (1,2). Chemical agents such as cytotoxic drugs, in particular of the alkylant class, and ionizing radiation may directly cause DSB (3-7).
It has been suggested that the number of DSB correlate with the expected effect of an anti-tumor treatment. Their quantitation may therefore be useful for monitoring cancer therapies, both with regard to their effectiveness as to adverse events (8,9).
Compared to the current post-hoc evaluation of antineoplastic therapy, such a monitoring tool would offer a number of advantages. Currently, dosages of both cytotoxic drugs and radiation therapies are determined empirically on large numbers of patients. Physical characteristics such as weight or height, or the combination thereof summarized in the body surface area, are the standard way to determine individual dosage. Not only is this approximation from a large cohort onto the individual patient a rather rough one. In addition, the dose steps to be tested in clinical trials usually lack an inherently biological rationale (10,11): starting from assumptions gained in non-human models, usually animal experiments, most drugs are tested in steps of multiples of a more or less arbitrary start dose. For example, bendamustine is usually given in doses of 60, 90, or 120 mg/m2 (12). It appears quite unlikely that exactly these steps and not some odd figures nearby or in between represent biologically optimal doses. Variability in both tumor biology and metabolism forecloses the same dose to have the same quantitative effect in any two patients.
Yet currently, there are only limited ways to adapt chemotherapy to a given disease and patient (10). Individual dose adjustments are possible only post-hoc, i.e., by dose reduction once adverse events have occurred—which is often too late to prevent serious and lasting damage or even death. Likewise, therapy failure most often is only recognized upon re-staging after a predefined number of months and therapy cycles, and the usual response to therapy failure is not an increase in dose, but a change to a second-line regimen.
In summary, although the therapeutic index of most anti-tumor drugs is notoriously small, actual dosage in the individual patient rather follows rules of thumb than precise measures, leading to therapy failure in the lower and potentially lethal adverse effects in the higher range of individual responsivity.
To solve this eminent clinical problem, we agree with other authors that personalized medicine is only feasible with the support of in vitro diagnostic testing (13). Here we investigate the feasibility of monitoring cellular effects in peripheral blood lymphocytes as a measure of cytotoxicity induced by the alkylating agent bendamustine, a commonly used drug in lymphoma therapy.
The localization and phosphorylation of H2AX histones (then termed γH2AX) in close proximity to DNA break sites are among the earliest events in the nuclear response to DNA DSB (7,14-16). Whether they are as specific for DSB as has been suggested by some authors is a matter of debate (17). Nonetheless, in a pragmatic view the number of γH2AX-histones appears to correlate well with the severity of DNA damage (17).
Hence, γH2AX may constitute an indicator for monitoring and controlling the intensity of cytotoxic therapy over time, predicting and ultimately balancing efficacy and toxicity as an approach to personalized therapy with conventional cancer drugs. In the particular case of lymphoma, a varying proportion of the investigated lymphocytes will also be circulating malignant cells. Therefore, we additionally hypothesize that the number of γH2AX foci per cell prior to therapy might be an indicator of the severity of the disease and thus be of prognostic value.
Manual fluorescence microscopy is a time-consuming and error prone method, especially for routine examination of patient samples, as it requires extensive training and is still fraught with high inter-investigator variability. Computerized image acquisition and analysis offers considerably higher throughput and objectivity (18). Moreover, such digital platforms can be combined with laboratory information systems for data integration, which is of high importance in clinical routine (19). The AKLIDES NUK® system (Medipan, Dahlewitz, Germany) allows for fast and reliable fluorescence analysis of up to 96 cell samples in a single run. The device processes immunofluorescent-stained cells or tissue and uses sophisticated software algorithms for image segmentation and analysis (Figure 1).

Here we present an exploratory study to elucidate in vitro the correlation between cell exposure to the cytotoxic agent bendamustine and DNA damage, using peripheral blood mononuclear cell (PBMC) samples from healthy donors, and the feasibility of using the automated AKLIDES NUK® system for routine determination of γH2AX foci (18).
Methods
All human subjects participated in this study with full written informed consent in accordance with the Helsinki declaration and federal and local laws, regulations and ethics guidelines. Ethical approval has been granted by the ethics committee of the Brandenburg physicians’ chamber [Landesärztekammer Brandenburg, document number S 15(a)/2015].
Sample collection and isolation of PBMC
A total of 12 volunteer donors (70% female, 30% male, age 25–60 yrs.) donated approximately 30 mL of venous blood each. The donor blood was obtained by a trained medical person through venous puncture of a cubital vein and aspiration of blood into a standardized EDTA sample container (Sarstedt, Nümbrecht, Germany). Fresh blood aliquots of 6.5 mL each were diluted 1:2 in phosphate buffered saline (PBS), layered over an equal volume of lymphocyte separating solution (LSM 1077, GE Healthcare, Solingen, Germany) into a 15 mL centrifugation tube and submitted to gradient centrifugation at 1,200 G for 20 min. After recovering the PBMC fraction, it was washed and re-suspended to a final density of 1.5×106 cells/500 µL in MEM + (GE Healthcare, Solingen, Germany) medium containing 10% fetal bovine serum.
Incubation of PBMC
PBMC suspension was seeded onto in a 24-well culture plate (Corning Inc., New York, USA) at 500 µL per sample and bendamustine (Sigma Aldrich, Taufkirchen, Germany) was added in various concentrations as stated in Results. These samples were subsequently incubated for 3 hours at 37 °C with 7% CO2 and saturated water vapor.
In all experiments, triplets of untreated cells from each donor served as negative controls (NCs) as DNA DSB may occur even in healthy persons due to genetic rearrangements, e.g., for the diversification of naturally occurring antibodies (2,21). In addition, another triplet for each donor was treated with bendamustine as positive control for the induction of DNA DSBs. This induced a comparable number of DSB as etoposide at a concentration of 5 µM (22).
γH2AX foci staining
Aliquots of 50 µL cell suspension were pipetted onto a Teflon-coated glass slide and left to rest for 10 minutes. A 2% formaldehyde solution in PBS was added as a fixative for 15 minutes at room temperature. The cell samples were washed three times in PBS, then permeabilized for 5 minutes with 0.2% Triton-X-100 in PBS with 1% bovine serum albumin (PBS-BSA) on ice and washed again in PBS-BSA as before.
Primary staining was performed with an anti-phospho-histone H2AX mouse monoclonal IgG primary antibody (Millipore, Schwalbach, Germany) diluted 1:1,000 in PBS-BSA. PBMC were incubated at 4 °C overnight and washed another three times for 10 minutes each in PBS-BSA. For secondary staining, cells were incubated with goat anti-mouse IgG antibody conjugated to Alexa Fluor 488 (Invitrogen, Darmstadt, Germany) 1:500 in PBS-BSA for 1 hour at 20 °C followed by three final washing steps in PBS. Using 40,6-diamidino-2-phenylindole (DAPI)-containing mounting medium (Medipan, Berlin/Dahlewitz, Germany), cells were covered and later sealed with a coverslip.
Automated fluorescence microscopy
The computer-mediated quantitation of the slides for γH2AX foci was carried out using the AKLIDES platform (Medipan, Berlin/Dahlewitz, Germany), allowing for fully automated image acquisition, analysis, and evaluation [for a detailed description of the system see (23,24)]. For detection of the stained cell nuclei a DAPI staining (blue) was used and γH2AX foci were analyzed in a FITC (green) channel. The following settings were used for all experiments: standard focus position, 6,050; standard exposure DAPI, 25 ms; standard exposure FITC, 800 ms; minimum and maximum cell diameter, 2 and 15 µm, respectively; and magnification, 60 fold. Foci were counted from a minimum of 20 cells per sample. The AKLIDES NUK® system reports the mean number of foci per cell.
Statistical analysis
Statistical analyses were based on the median (Md) as a robust measure for central tendency. Of note, means and Mds were comparable as assessed by Pearson correlation analysis [r=0.99, (95% CI: 0.990, 0.997) t=65.28, df =47, P<2.2e-16, adjusted R2 =0.99, P<2.2e-16]. Accordingly, the difference between the 25th and the 75th percentile (interquartile range, IQR) was used as measure of statistical dispersion. Each value was calculated from triplicate measurements of each sample. Pairs of samples were compared as indicated in the following paragraphs. Statistical analyses were performed using the RKWard (v. 0.6.9z+0.7.0+devel1) integrated development environment (25) and Python 3.6 (https://www.python.org/) with the IPython environment for scientific computing (26).
Results
Dose calculations
For the in vitro model, the standard doses from the rituximab-bendamustine immune-chemotherapy protocol were converted into the corresponding serum concentrations expected immediately after application. Two alternative calculations, standardized and individualized, were used to convert the clinically applied dosage into a model dose per sample:
The standardized calculation assumed a 1.75 m and 75 kg “standard person”, resulting in a body surface area according to the Mosteller formula of 1.9 m2, which was then used for samples from all donors (27). For the individualized dose calculation, the actual height and weight of the donor were used to calculate the surface area (1.81±0.14 m2).
From the estimated blood volume in milliliters, calculated as weight (kg) ×75 for men and weight (kg) ×65 for women, the clinical dose per milliliter of blood was calculated. Further assuming a normal lymphocyte count of 3×106 lymphocytes per milliliter of blood, for the assay the PBMC from the donors were adjusted to 3×106 per well, and the clinical dose per milliliter of blood as calculated above was applied to each well.
Baseline numbers of γH2AX foci per cell
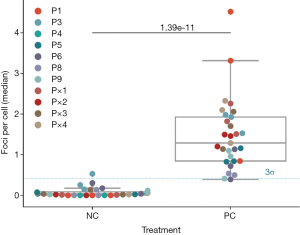
As NCs, donor PBMC were prepared and mock-incubated without drug as described in Methods. The baseline γH2AX foci count analysis was conducted with 10 out of the 12 volunteer donors, since two donor samples had to be excluded due to technical issues (insufficient isolation yield and artifacts during measurement). Figure 2 shows the Md number of foci per cell for each donor before and after treatment for 11 donors (P1–P9 and P×1–P×4). As empirical estimate we set a 99.7% threshold (µ+3σ) of 0.41 foci per cell for both groups based on the 68–95–99.7 rule. Donor P3 seemed to have higher foci counts per cell than P1, P4, P5, P6, P8 and P9 (Figure 3A). Donor P7 had higher baseline γH2AX foci counts per cell than the other donors and therefore was not included in the next analysis steps (Figure 3B). The difference between the untreated [0.04, IQR (0.08) foci per cell] and treated donor PBMCs [1.29, IQR (1.08) foci per cell] was significant (U=3.0, P=1.39e-11) as assessed by the Mann-Whitney rank test. The analysis was continued with the donors P1, P3, P4, P5, P6, P8 and P9 since they were in the range of the 99.7% threshold.
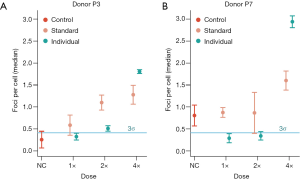
Standardized and individualized dose calculations
The standardized therapy-equivalent dose of bendamustine per well, based upon a 175 cm and 75 kg person, was calculated as 20.4 µg. Adjusting for the actual height and weight of the donors to calculate the individualized doses, the average was 23.0 µg with a range of 19.4 to 27.1 µg. The higher mean concentration reflects the fact that the donors were mostly taller than the 175 cm assumed for a standard person.
Exposure to bendamustine
Donor PBMCs were incubated with bendamustine in a dose per 3,000 cells equivalent to the clinically used therapeutic dosage of 120 mg/m2, applying either the standardized or individualized dose calculation as described above. To determine dose-dependent responses, an incremental series of single, double and fourfold standard dose were applied (Figure 3).
The number of foci per cell of the treated groups (standard dosages, individual dosages) was compared to the untreated control by the Kruskal-Wallis test to test if there is in general an effect of the treatment. Both the standard dosages (χ2=10.74, df=3, P=0.01323) and the individual dosages (χ2=20.22, df=3, P=0.000153) were significantly different. Thus, in comparison to the NC, bendamustine induced a significant increase in the number of foci per cell, both with the standard (Figure 4A) and individualized treatment (Figure 4B). Thus, the comparison over all donors tested showed a positive relation between bendamustine concentration and number of γH2AX foci.
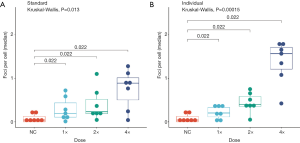
Individualized 4× doses were on average higher (P=0.036) than the standard dose when compared pair-wise by Wilcoxon rank sum test. The individual dosages showed a tendency for a higher foci number per cell. The Md of the highest dosages (4×) for the donors P1, P3, P4, P5, P6, P8 and P9 resulted in 0.89, IQR (0.51) foci per cell with standardized and in 1.57, IQR (0.5) foci per cell [~1.75, IQR (0.63) fold increase] with individualized dose calculation. This overall result held true upon reviewing the individual donors for whom titration series were available (Figure 4). As determined according to (28), the P value obtained in the Wilcoxon test (P=0.022) was more extreme than in 99.2% of all possible P values in the standard dosages and 100% of all possible P values in the individual dosages. This amplifies the significance of the findings. Compared to standardized dosage, the individualized calculation provided more coherent results. Analyzing the relation between body surface area and γH2AX foci, a negative trend (i.e., larger body surface is corresponds to lower number of γH2AX foci per cell) was seen under all treatment conditions, which appeared stronger with individualized dosage, while the Pearson product moment correlation coefficient (Pearson’s R) test for association between paired samples revealed no significant correlation (Table 1).
Table 1
| Dose | R2 | m | n | r | P(r) | 95% CI | Significant | ||
|---|---|---|---|---|---|---|---|---|---|
| Standard dose calculation | NC | 0.32 | −0.493 | 0.992 | −0.56 | 0.00799 | −0.8 | −0.172 | n |
| 1× | 0.42 | −1.6 | 3.22 | −0.65 | 0.00191 | −0.85 | −0.292 | n | |
| 2× | 0.38 | −1.42 | 2.96 | −0.62 | 0.00292 | −0.83 | −0.252 | n | |
| 4× | 0.18 | −1.35 | 3.23 | −0.43 | 0.0524 | −0.73 | 0.003 | n | |
| Individual dose calculation | NC | 0.32 | −0.493 | 0.992 | −0.56 | 0.00799 | −0.8 | −0.172 | n |
| 1× | 0.36 | −0.696 | 1.48 | −0.6 | 0.00529 | −0.82 | −0.212 | n | |
| 2× | 0.67 | −1.36 | 2.93 | −0.82 | 6.53E-06 | −0.92 | −0.593 | n | |
| 4× | 0.38 | −2.08 | 5.08 | −0.62 | 0.00843 | −0.85 | −0.193 | n | |
A linear model was used to test the relation between the body surface area of the donors [1.8, IQR (0.15) m2, P1=2.13, P3=1.67, P4=1.9, P5=1.8, P6=1.67, P8=1.75, P9=1.82/m2] and the formation of γH2AX foci after treatment with bendamustine. R2, coefficient of determination; m and n, slope and intercept of the linear regression model; r, Pearson’s correlation coefficient; P(r), P value for Pearson’s correlation coefficient; 95% CI, 95% confidence interval of Pearson’s correlation coefficient; Significant, if P(r) ≤0.01, then correlations were considered significant.
Comparing baseline and bendamustine-induced γH2AX foci counts between donors, a large variability was found (Figure 4). With both standardized and individualized dosage, we observed a dose dependent effect (Figure 5). Yet only donor P4, P5, P8 and P9 signify that there is indeed a marked increase (Figure 5B). Therefore, we tested if the dose-response relationship was linear and found this true for a number of donors with both the standardized and the individualized bendamustine treatment (Table 2).
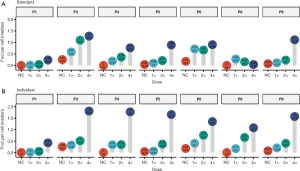
Table 2
| Donor | R2 | m | n | r | P(r) | 95% CI | Significant | ||
|---|---|---|---|---|---|---|---|---|---|
| Standard dose calculation | P1 | 0.79 | 0.071 | −0.045 | 0.89 | 0.000118 | 0.64 | 0.968 | y |
| P3 | 0.7 | 0.218 | 0.422 | 0.84 | 0.000647 | 0.51 | 0.954 | y | |
| P4 | 0.92 | 0.173 | 0.022 | 0.96 | 7.85E-07 | 0.86 | 0.989 | y | |
| P5 | 0.78 | 0.224 | −0.075 | 0.88 | 0.000138 | 0.63 | 0.967 | y | |
| P6 | 0.31 | 0.155 | 0.384 | 0.56 | 0.0596 | −0.02 | 0.857 | n | |
| P7 | 0.47 | 0.198 | 0.694 | 0.69 | 0.0279 | 0.1 | 0.919 | n | |
| P8 | 0.01 | −0.01 | 0.151 | −0.12 | 0.723 | −0.67 | 0.516 | n | |
| P9 | 0.74 | 0.31 | −0.14 | 0.86 | 0.000355 | 0.56 | 0.959 | y | |
| Individual dose calculation | P1 | 0.45 | 0.102 | −0.065 | 0.67 | 0.0228 | 0.13 | 0.907 | n |
| P3 | 0.85 | 0.385 | 0.061 | 0.92 | 2.08E-05 | 0.74 | 0.978 | y | |
| P4 | 0.8 | 0.368 | −0.091 | 0.9 | 0.0011 | 0.57 | 0.978 | y | |
| P5 | 0.86 | 0.403 | −0.157 | 0.93 | 4.16E-05 | 0.74 | 0.981 | y | |
| P6 | 0.88 | 0.296 | 0.18 | 0.94 | 5.66E-06 | 0.79 | 0.983 | y | |
| P7 | 0.67 | 0.659 | −0.141 | 0.82 | 0.0022 | 0.42 | 0.951 | y | |
| P8 | 0.83 | 0.28 | −0.001 | 0.91 | 3.66E-05 | 0.71 | 0.975 | y | |
| P9 | 0.83 | 0.36 | −0.101 | 0.91 | 3.61E-05 | 0.71 | 0.975 | y | |
A linear model was used to test the relationship between the dose of bendamustine and number of foci per cell. R2, coefficient of determination; m and n, slope and intercept of the linear regression model; r, Pearson’ correlation coefficient; P(r), P value for Pearson’s correlation coefficient; 95% CI, 95% confidence interval of Pearson’s correlation coefficient; Significant, if r≥0.8 P(r) ≤0.01, then correlations were considered significant.
Discussion
This exploratory study was designed to test the feasibility of detecting chemotherapy-induced double-strand breaks represented by γH2AX foci in human blood in an automated manner using the AKLIDES NUK® immunofluorescence analysis platform. While it ultimately aims at developing a real-time method for assessing cytotoxic effects in lymphoma patients, in itself it is decidedly an exploratory, technical feasibility study. Hence, conclusions regarding the in vivo effect of chemotherapy cannot be drawn from it, and only the in vitro use of bendamustine and its measurability by the experiments are being described. This also means that the proposed in vitro model does not accurately resemble the dose-effect-relations to be expected in vivo.
In solid, non-leukemic lymphoma, neoplastic lymphocytes require sophisticated techniques for detection and do not normally form a significant proportion of PBMCs (29). Hence, the rationale behind measuring PBMCs for an assessment of both therapeutic and adverse effects is to measure bystander lymphocytes and to use their degree of damage as an inverse surrogate for the absorption of drug effect by the unknown quantity of malignant cells. This study was designed to investigate the feasibility of the measurement methodology. Whether this will be clinically valid as a diagnostic tool is the subject of ongoing investigations.
Accordingly, the calculations of dose extrapolations are solely meant to guide the experiments in terms of orders of magnitude, not as exact models. The blood concentrations of chemotherapy vary vastly among individual patients due to differences in metabolism and drug clearance, which is the very point of a real-time efficacy assessment. By definition, this can only be addressed in real patients who have been administered the treatment.
As such, the in vitro use of bendamustine is intended to answer two questions: does bendamustine exert any effect on PBMC that can be measured by the γH2AX assay, and if so, is this a quantitative phenomenon? The authors are completely aware that this in vitro model does not in any way accurately resemble the dose-effect-relations to be expected in vivo for normal tissue toxicity (adverse events) and tumor response. To this end, we examined (I) whether this automated analysis technology will be suitable for routine use on clinical samples with regard to pre-analytic requirements and measurement reliability; (II) how consistent (or variable) γH2AX measurements are and which factors contribute to variation; and (III) whether measurements of γH2AX qualify for monitoring dose-dependent biological effects of bendamustine.
Briefly, our results lead to three conclusions:
- Automated routine measurement of γH2AX foci in buffy coat isolated PBMCs from human blood samples is practically and scientifically feasible;
- Baseline values of γH2AX foci appear to vary considerable among individuals, and so do maximum values after PBMC incubation with the cytotoxic drug bendamustine;
- In vitro incubation of donor PBMCs with bendamustine consistently leads to a dose-dependent increase in γH2AX foci in all individuals examined.
These brief statements qualify for further comments.
Regarding statement one, it is not a trivial finding that PBMC preparation by density gradient yields reproducible fluorescence readings resulting from a complex nuclear process such as histone phosphorylation and translocation. Results not demonstrated here have also shown that freezing at −80 °C and thawing of buffy coat preparations does not significantly alter γH2AX readings. This result is therefore encouraging for future clinical studies.
Regarding the practical application in future cohort studies or even clinical routine use, the technology used here appears highly useful since most steps are automatized. In principle, large sample quantities can be processed in due time for clinical decision making. Beyond this, the scientific depth of each analysis is inherently superior to visual microscopic examination, as the automated system captures multiple z-planes at 1 µm distances each from the same region of interest to spatially identify all foci in each cell (18), and images can be stored and reanalyzed as needed.
The inter-individual variation of γH2AX baseline foci that we observed in this exploratory study may pose an obstacle to population-based validations and the determination of prognostic threshold values. Like any other biomarker, γH2AX has a biological variation, which may be predictable, cyclical or even random over the entire life span. To account for such differences between individuals is not trivial (30). Both biological and technical sources of variation need to be taken into consideration during the planned follow-up study with a larger cohort.
Among the technical sources of variability, we expect issues of pre-analytic sample processing. This is a common problem in laboratory medicine (31) and can be minimized by organizational measures such as establishment of standard operating procedures and personal training. Other technical challenges we encountered, such as dry-outs of samples that rendered further analyses invalid, also fall into this category (Figure 6).
Of relevant concern, however, are intrinsic biological sources of variability, which can only be hypothesized but not further examined on the basis of the small sample size examined here.
Still, it appears likely that the variability of baseline values we found is not an erroneous laboratory artifact but a real observation, as it concurs with the finding of large inter-individual variation in a study correlating γH2AX-foci in hematopoietic stem cells with biological ageing (32). This is not a trivial finding, either, as γH2AX has been suggested not only as a marker for cytotoxic (33) or radiotherapy effect and toxicity (9,34,35), but also for cancer prognosis in solid tumors (36,37). However, the current body of evidence does not foreclose a potential use of DSB-analysis for prognostic assessments, as we cannot tell yet whether the observed variation is a random phenomenon (possibly varying swiftly over time) or a meaningful signal.
Anecdotal evidence from our donor group suggests the latter with donor P7 (Figure 3): DNA DSB have been described as a response to a number of stressors, including psychosocial factors, and in an additional interview this donor turned out to have experienced a singular traumatic and a number of additional psychically and physically stressful events only recently before blood sampling.
Thus, whether population-based prognostic estimates will be possible in newly diagnosed lymphoma patients remains to be investigated in a clinical study with a large number of patients and controls.
The same applies to the use of γH2AX measurements for therapy monitoring. Here, too, we observed strong inter-individual variability, and while each individual showed some dose-dependent response, no γH2AX reading or γH2AX increase over baseline correlated to a certain dose range across the whole population. Again, whether this variation is a random effect or actually constitutes the basis for meaningful individual predictions of response and toxicity is beyond the scope of this practical exploratory study.
Bendamustine was selected for this exploratory study not because the authors deemed it particularly suited for their assay, but because of its relevance in lymphoma therapy. Finding an optimal biomarker for bendamustine response is yet an open objective. Primarily described as a DNA cross-linking alkylating agent, it has been questioned whether bendamustine induces dose-dependent H2AX phosphorylation at all (38,39). Yet it differs from other alkylating agents in the variety of its mechanisms of action. Even more so, the finding of dose dependent individual γH2AX responses to bendamustine in itself is a relevant finding: in the majority of samples the γH2AX response was dose-dependent even at small concentrations, which suggests that the assay is indicative for a reaction upon treatment at least in vitro.
This work has been deliberately limited to γH2AX as a well-described biomarker of DNA DSBs (1). Of course, a number of other biomarkers, such as the MYC proto-oncogene (c-Myc), tumor suppressor p53-binding protein 1 (53BP1), serine/threonine-protein kinase (ATR), signal transducer and activator of transcription 3 (STAT3), apoptosis analysis and nucleus size may improve and enhance this approach to cell-based prediction and therapy monitoring (40). These parameters, each alone or in combinations, can easily be included in future investigations based on the AKLIDES platform.
We agree with Zhang (41), who stated that “laboratory medicine aims to provide tests to guide clinical decision making”. Therefore, this preliminary work is meant to set the basis for a larger study in lymphoma patients to validate γH2AX as a potential prognostic or predictive marker for disease risk, adverse events, and treatment outcome.
Based on these data, the authors thus decided to follow this concept in a pilot clinical study in patients newly diagnosed with malignant B-cellular lymphoma.
Acknowledgments
We would like to thank Michał Burdukiewicz for his input on the data analysis.
Funding: This work has in part been funded by the “Gesundheitscampus Brandenburg - Konsequenzen der alterassoziierten Zell - und Organfunktionen” initiative of the Brandenburgian Ministry of Science, Research and Culture (MWFK) and the InnoProfile-Transfer 03 IPT 611X project (Federal Ministry of Education and Research).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dirk Roggenbuck) for the series “DNA Damage Assessment for Precision Medicine” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.04.10). The series “DNA Damage Assessment for Precision Medicine” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval has been granted by the ethics committee of the Brandenburg physicians’ chamber [Landesärztekammer Brandenburg, document number S 15(a)/2015]. All human subjects participated in this study with full written informed consent in accordance with the Declaration of Helsinki (as revised in 2013) and federal and local laws, regulations and ethics guidelines.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bonner WM, Redon CE, Dickey JS, et al. γH2AX and cancer. Nat Rev Cancer 2008;8:957-67. [Crossref] [PubMed]
- Mehta A, Haber JE. Sources of DNA Double-Strand Breaks and Models of Recombinational DNA Repair. Cold Spring Harb Perspect Biol 2014;6:a016428 [Crossref] [PubMed]
- Clouaire T, Marnef A, Legube G. Taming Tricky DSBs: ATM on duty. DNA Repair (Amst) 2017;56:84-91. [Crossref] [PubMed]
- Watts FZ. Repair of DNA Double-Strand Breaks in Heterochromatin. Biomolecules 2016;6. [PubMed]
- Rube CE, Fricke A, Schneider R, et al. DNA repair alterations in children with pediatric malignancies: novel opportunities to identify patients at risk for high-grade toxicities. Int J Radiat Oncol Biol Phys 2010;78:359-69. [Crossref] [PubMed]
- Giunta S, Jackson SP. Give me a break, but not in mitosis: the mitotic DNA damage response marks DNA double-strand breaks with early signaling events. Cell Cycle 2011;10:1215-21. [Crossref] [PubMed]
- Reddig A, Rübe CE, Rödiger S, et al. DNA damage assessment and potential applications in laboratory diagnostics and precision medicine. J Lab Precis Med 2018;3:31. [Crossref]
- Andrievski A, Wilkins RC. The response of gamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. Int J Radiat Biol 2009;85:369-76. [Crossref] [PubMed]
- Bourton EC, Plowman PN, Smith D, et al. Prolonged expression of the gamma-H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int J Cancer 2011;129:2928-34. [Crossref] [PubMed]
- Gao B, Klumpen HJ, Gurney H. Dose calculation of anticancer drugs. Expert Opin Drug Metab Toxicol 2008;4:1307-19. [Crossref] [PubMed]
- van Warmerdam LJ. Tailor-made chemotherapy for cancer patients. Neth J Med 1997;51:30-5. [Crossref] [PubMed]
- Hoy SM. Bendamustine: a review of its use in the management of chronic lymphocytic leukaemia, rituximab-refractory indolent non-Hodgkin's lymphoma and multiple myeloma. Drugs 2012;72:1929-50. [Crossref] [PubMed]
- Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7. [Crossref]
- Sedelnikova OA, Rogakou EP, Panyutin IG, et al. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res 2002;158:486-92. [Crossref] [PubMed]
- Bouquet F, Muller C, Salles B. The loss of gammaH2AX signal is a marker of DNA double strand breaks repair only at low levels of DNA damage. Cell Cycle 2006;5:1116-22. [Crossref] [PubMed]
- de Feraudy S, Revet I, Bezrookove V, et al. A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci U S A 2010;107:6870-5. [Crossref] [PubMed]
- Löbrich M, Shibata A, Beucher A, et al. gammaH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle 2010;9:662-9. [Crossref] [PubMed]
- Willitzki A, Lorenz S, Hiemann R, et al. Fully automated analysis of chemically induced gammaH2AX foci in human peripheral blood mononuclear cells by indirect immunofluorescence. Cytometry A 2013;83:1017-26. [Crossref] [PubMed]
- McCudden CR, Henderson MPA. Laboratory information system data extraction and re-use: opportunities and challenges. J Lab Precis Med 2017;2:81. [Crossref]
- Ruhe M, Schierack P, Dammermann W, et al. γH2AX and related biomarker in elderly people with emphasis on diffuse large B-cell lymphoma. F1000Res 2017;6:2135.
- Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis 2002;23:687-96. [Crossref] [PubMed]
- Reddig A, Lorenz S, Hiemann R, et al. Assessment of modulated cytostatic drug resistance by automated γH2AX analysis. Cytometry A 2015;87:724-32. [Crossref] [PubMed]
- Rödiger S, Schierack P, Böhm A, et al. A highly versatile microscope imaging technology platform for the multiplex real-time detection of biomolecules and autoimmune antibodies. Adv Biochem Eng Biotechnol 2013;133:35-74. [Crossref] [PubMed]
- Willitzki A, Hiemann R, Peters V, et al. New platform technology for comprehensive serological diagnostics of autoimmune diseases. Clin Dev Immunol 2012;2012:284740.
- Rödiger S, Friedrichsmeier T, Kapat P, et al. RKWard: a comprehensive graphical user interface and integrated development environment for statistical analysis with R. J Stat Softw 2012;49:1-34. [Crossref]
- Pérez F, Granger BE. IPython: a System for Interactive Scientific Computing. IEEE 2007;9:21-9.
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098. [Crossref] [PubMed]
- Pitman EJG. Significance Tests Which May be Applied to Samples from any Populations. II. The Correlation Coefficient Test. J R Stat Soc Series 1937;4:225-32.
- Chase ML, Armand P. Minimal residual disease in non-Hodgkin lymphoma - current applications and future directions. Br J Haematol 2018;180:177-88. [Crossref] [PubMed]
- Fraser CG. Biological variation: a rapidly evolving aspect of laboratory medicine. J Lab Precis Med 2017;2:35. [Crossref]
- Wiwanitkit V. Errors in medical laboratory but still forgotten. J Lab Precis Med 2017; 2017;2:65.
- Rübe CE, Fricke A, Widmann TA, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One 2011;6:e17487 [Crossref] [PubMed]
- Yu J, Qiu S, Ge Q, et al. A novel SAHA-bendamustine hybrid induces apoptosis of leukemia cells. Oncotarget 2015;6:20121-31. [PubMed]
- Sak A, Stuschke M. Use of gammaH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin Radiat Oncol 2010;20:223-31. [Crossref] [PubMed]
- Shah K, Boghozian RA, Kartsonaki C, et al. gammaH2AX expression in cytological specimens as a biomarker of response to radiotherapy in solid malignancies. Diagn Cytopathol 2016;44:141-6. [Crossref] [PubMed]
- Mei L, Hu Q, Peng J, et al. Phospho-histone H2AX is a diagnostic and prognostic marker for epithelial ovarian cancer. Int J Clin Exp Pathol 2015;8:5597-602. [PubMed]
- Palla VV, Karaolanis G, Katafigiotis I, et al. gamma-H2AX: Can it be established as a classical cancer prognostic factor? Tumour Biol 2017;39:1010428317695931 [Crossref] [PubMed]
- El-Mabhouh AA, Ayres ML, Shpall EJ, et al. Evaluation of bendamustine in combination with fludarabine in primary chronic lymphocytic leukemia cells. Blood 2014;123:3780-9. [Crossref] [PubMed]
- Jain N, Balakrishnan K, Ferrajoli A, et al. A phase I-II trial of fludarabine, bendamustine and rituximab (FBR) in previously treated patients with CLL. Oncotarget 2017;8:22104-12. [Crossref] [PubMed]
- Zhang Z. The role of big-data in clinical studies in laboratory medicine. J Lab Precis Med 2017;2:34. [Crossref]
Cite this article as: Rödiger S, Liefold M, Ruhe M, Reinwald M, Beck E, Deckert PM. Quantification of DNA double-strand breaks in peripheral blood mononuclear cells from healthy donors exposed to bendamustine by an automated γH2AX assay—an exploratory study. J Lab Precis Med 2018;3:47.


