Hemostasis practice: state-of-the-art
Introduction
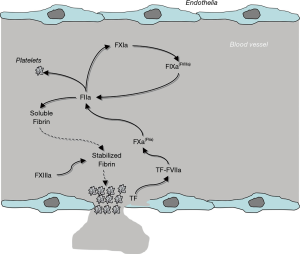
Physiological hemostasis is conventionally defined as a complex biological pathway aimed at arresting blood leakage from injured venous and arterial vessels, and concomitantly preventing excessive or unwarranted blood clotting when these structures are undamaged (1). Albeit a thorough description of physiological hemostasis shall be omitted here due to space constraints, some important elements need to be described. Briefly, hemostasis is typically divided into primary and secondary, the former mainly encompassing vasoconstriction and generation of a preliminary and labile platelet plug, the latter being represented by ‘blood coagulation’ (2). A third biological mechanism, conventionally called fibrinolysis, integrates into hemostasis by dissolving blood clots once these are no longer necessary (i.e., once the haemorrhage has been definitely arrested) (1) (Figure 1).
Physiological hemostasis
A blood vessel injury is suddenly accompanied by the onset of an arterial vasoconstriction, aimed at reducing the size of the vessels and thereby the potential amount of blood that may leak outside of the vessel itself, a process then followed by activation of blood platelets neighbouring the injury. Platelets can be activated by either direct contact with subendothelial surfaces (i.e., collagen) that become exposed after endothelial injury, or by thrombin generated by blood coagulation, as described elsewhere in this article. Once activated, platelets undergo a series of biochemical and structural changes, mainly entailing shape changes (to facilitate platelet-to-platelet interaction), adhesion to subendothelial structures, and aggregation (i.e., platelet-to-platelet binding) (1) (Figure 1). Provided that platelet number and function are preserved, this former mechanism enables the generation of a preliminary plug, which contributes to slow down or completely arrest the leakage of blood outside the vessel. Nevertheless, the stability of this plug is strongly challenged by blood flow and shear stress, which may both contribute to gradually dissolve the clot, thus exposing the patient to the risk of subsequent hemorrhage (3).
The stabilization of the platelet plug, which is hence necessary to efficiently counteract bleeding, is mediated by the activation of blood coagulation, whose ultimate scope is generating a sufficient amount of fibrin strands to act like mortar for the bricks (i.e., the platelets). Unlike former theories, the modern and “unified” vision of blood coagulation no longer encompasses three different pathways (i.e., the so-called intrinsic, extrinsic and common pathways), but considers blood coagulation as an integrated biological system, primarily assembled on cellular structures, starting from release of tissue factor (TF) from damaged endothelia, and proceeding further with sequential activation of many inactive clotting proteins, up to fibrin generation1) (Figure 1). Rapidly upon release into the blood stream, TF associates with factor (F) VII, thus generating a dimeric protein complex which is auto-catalytic, but is also capable of activating FX, the ensuing factor in the coagulation cascade. Combined with activated (a) FV to form the so-called prothrombinase complex, FXa catalyzes the conversion of prothrombin (FII) to thrombin (FIIa), which consequently catalyzes the conversion of fibrinogen to fibrin. Whilst this straightforward biological pathway is actually effective to generate fibrin, the overall amount of fibrin produced here is largely ineffective to ensure clot stability (i.e., between 3–5% of the total amount needed). It is at this point that the clotting factors of the formerly known “intrinsic” pathway come into play (1). Thrombin not only is capable of activating platelets and transforming fibrinogen into fibrin (Figure 1), but also catalyzes the conversion of FXI into FXIa. This latter protein in turns activates FIX to FIXa which, in conjunction with its cofactor FVIIIa, promotes the conversion of FX into FXa, thus closing the “circle” and bringing back the pathway to the prothrombinase complex. This specific mechanism, which has been for long identified as the “intrinsic” pathway, is now more conveniently known as “thrombin burst”, which enables (provided that concentration and activity of all clotting factors are preserved) the generation of the necessary amount of fibrin for more efficiently stabilizing the platelet plug after FXIIIa-mediated polymerization of soluble fibrin (Figure 1) (1). Under physiological conditions, the blood clot is then gradually dissolved by the activity of the fibrinolytic system (i.e., by plasmin, converted from plasminogen by tissue plasminogen activator), with release into the circulation of the co-called fibrin/fibrinogen degradation products (FDPs), including D-dimer (1).
The hemostatic balance is then finely modulated by a number of endogenous inhibitors, which are capable to neutralize one or more clotting factors, and are mostly represented by antithrombin (whose function is enormously magnified by heparin and other glycosaminoglycans), the protein C-protein S pathway, TF pathway inhibitor (TFPI, formerly known as extrinsic pathway inhibitor), thrombin activatable fibrinolysis inhibitor (TAFI) and plasminogen activator inhibitor-1 (PAI-1) (Table 1) (4).
Table 1
| Inhibitor | Target protein |
|---|---|
| Tissue factor pathway inhibitor (TFPI) | Tissue factor |
| Antithrombin | Factor II and X |
| Protein C-protein S | Factor V and VIII |
| Thrombin activatable fibrinolysis inhibitor (TAFI) | Fibrin |
| Plasminogen activator inhibitor-1 (PAI-1) | Tissue plasminogen activator (t-PA) |
Integrated approach to hemostasis disturbances
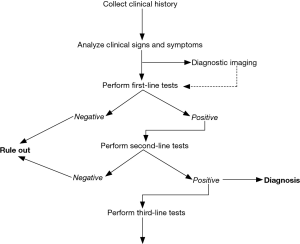
In accordance with the earlier definition of hemostasis, disorders of this biological system are typically classified as being either hemorrhagic (i.e., relatively insufficient clotting) or thrombotic (i.e., relatively disproportionate clotting). Regardless of the nature and the underlying causes, the diagnostic approach to patients with hemostasis disorders must inevitably involve an accurate collection of clinical history, physical examination, and results of an appropriate number and type of laboratory investigations (Figure 2) (1,4-7). Clinical history is indeed the mainstay for an accurate diagnosis, since in many cases it provides essential information to distinguish between the inherited or acquired nature of bleeding or thrombosis. Signs and symptoms are also crucial. Albeit the clinical distinction between disorders of primary and secondary hemostasis is not always so straightforward, especially for certain conditions such as von Willebrand disease (VWD), the defects of primary hemostasis are more often accompanied by instantaneous and more superficial bleeding, whilst those of the secondary hemostasis are more typically delayed and encompass profound haemorrhages (i.e., in muscles, joints, brain, internal organs) (5). Regarding venous thromboembolism (VTE), even in such cases the clinics is fundamental, since both deep vein thrombosis (DVT) and pulmonary embolism (PE) may be characterized by a kaleidoscope of suggestive symptoms, which may also lead to highly unfavourable consequences (up to death) when left untreated (8). Diagnostic imaging may then be useful, especially for revealing the presence of clots in peripheral veins or pulmonary arteries, but also for identifying the site and severity of haemorrhages, especially when these involve internal organs. Last but not least, the contribution of laboratory hemostasis is now unavoidable for the screening (i.e., pre-operative testing), diagnosis and therapeutic monitoring of both hemorrhagic and thrombotic disorders (9).
A hierarchic classification of laboratory investigations
Squeezed between a continuously expanding volume of tests and an even more often concerning shortage of public funding (10,11), clinical laboratories are now fully committed to quality and appropriateness, which not only would contribute to the sustainability of the healthcare systems, but will also prevent the generation of potentially avoidable harms to the patients (i.e., false positive test results generated by unnecessary or unwarranted testing) (12). Since the list of potentially useful hemostasis tests is continuously expanding, the most reliable strategy to contain inappropriateness entails developing a reasonable priority of eligible tests, which can hence be arbitrarily classified as first-line (i.e., screening), second-line (i.e., for the etiological diagnosis) and third-line (i.e., for biochemical or even molecular characterization) analyses. A reliable example of how hemostasis tests could be classified for optimizing resources and improving the diagnostic and therapeutic reasoning is summarized in Table 2. This possible classification actually mirrors that of laboratory services, which are now increasingly organized in “spoke” (i.e., peripheral), “hub” (i.e., core) and reference facilities within the same geographical area (13), and takes also great advantage from a kaleidoscope of technological innovations occurred in this specific branch of laboratory testing (14-16).
Table 2
| Classification | Setting | Hemorrhagic disorders | Thrombotic disorders |
|---|---|---|---|
| First-line | All clinical laboratories | • Prothrombin time (PT) | • D-dimer |
| • Activated partial thromboplastin time (APTT) | • Prothrombin time (PT) | ||
| • Fibrinogen | |||
| • Platelet count | |||
| • Platelet function screening | |||
| Second-line | “Hub” laboratories | • Mixing test | • Inhibitors deficiency (clotting or chromogenic activity, concentration) |
| • Clotting factors deficiency (clotting activity) | • Activated protein C resistance (APCr) | ||
| • Titration of inhibitors | • Lupus anticoagulants (LAC) | ||
| • von Willebrand disease testing | • Anticardiolipin antibodies | ||
| • Platelet aggregation studies | • Molecular testing (SNPs identification) | ||
| • Molecular testing (SNPs identification) | • Activity of direct oral anticoagulants (DOACs) | ||
| Third-line | Reference hemostasis laboratories | • Clotting factors deficiency (chromogenic activity or concentration) | • Concentration of direct oral anticoagulants (DOACs) |
| • Cytofluorometry | • Molecular testing (gene sequencing) | ||
| • Molecular testing (gene sequencing) | |||
| • Thrombin generation |
SNP, single nucleotide polymorphism
Unlike other areas of laboratory medicine (17), standardization, and even harmonization, are not always met for hemostasis testing (18). More specifically, albeit an acceptable degree of harmonization has now been achieved for certain tests such as PT and APTT, there is still evidence that other tests such as D-dimer and von Willebrand factor (VWF) testing are still plagued by major inconsistency. Many ongoing supranational initiatives are underway, with the obvious expectation that a better degree of harmonization shall be soon achieved (19,20).
Conclusions
There is now incontrovertible evidence that laboratory tests are integral to the diagnostic reasoning and to the managed care of patients with hemostasis disturbances, both haemorrhagic and thrombotic. Nevertheless, several important issues still plague this important branch of laboratory medicine (Table 3), including the relatively modest knowledge that many laboratory professionals have of hemostasis in health and disease, the unacceptable heterogeneity of available diagnostic algorithms for both diagnosis (21) and therapeutic management of hemostatic diseases (22), the accurate definition of reference ranges (23), the identification and communication of critical values (24), as well as the still unsatisfactory harmonization of some preanalytical (25), analytical (18) and postanalytical (26) procedures.
Table 3
| Poor knowledge of hemostasis in health and disease |
| Heterogeneity of available guidelines for diagnosis and therapeutic management |
| Inaccurate definition of reference ranges |
| Identification and communication of critical values |
| Harmonization of preanalytical, analytical and postanalytical procedures |
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Laboratory Medicine-25 Years on”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.07.07). The series “Laboratory Medicine-25 Years on” was commissioned by the editorial office without any funding or sponsorship. Giuseppe Lippi served as an unpaid Guest Editor of the series and serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Emmanuel J Favaloro serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from April 2017 to March 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Favaloro EJ. Laboratory hemostasis: milestones in Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med 2013;51:91-7. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med 2018;56:1035-45. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Venous and Arterial Thromboses: Two Sides of the Same Coin? Semin Thromb Hemost 2018;44:239-48. [Crossref] [PubMed]
- Lippi G, Franchini M, Guidi GC. Diagnostic approach to inherited bleeding disorders. Clin Chem Lab Med 2007;45:2-12. [Crossref] [PubMed]
- Lippi G, Franchini M, Favaloro EJ. Diagnostics of Inherited Bleeding Disorders of Secondary Hemostasis: An Easy Guide for Routine Clinical Laboratories. Semin Thromb Hemost 2016;42:471-7. [Crossref] [PubMed]
- Lippi G, Pasalic L, Favaloro EJ. Detection of mild inherited disorders of blood coagulation: current options and personal recommendations. Expert Rev Hematol 2015;8:527-42. [Crossref] [PubMed]
- Lippi G, Danese E, Favaloro EJ, et al. Diagnostics in venous thromboembolism: from origin to future prospects. Semin Thromb Hemost 2015;41:374-81. [Crossref] [PubMed]
- Lippi G, Franchini M, Targher G, et al. Help me, Doctor! My D-dimer is raised. Ann Med 2008;40:594-605. [Crossref] [PubMed]
- Bonar RA, Lippi G, Favaloro EJ. Overview of Hemostasis and Thrombosis and Contribution of Laboratory Testing to Diagnosis and Management of Hemostasis and Thrombosis Disorders. Methods Mol Biol 2017;1646:3-27. [Crossref] [PubMed]
- Lippi G, Plebani M. The add value of laboratory diagnostics: the many reasons why decision-makers should actually care. J Lab Precis Med 2017;2:100. [Crossref]
- Lippi G. Weighting healthcare efficiency against available resources: value is the goal. Diagnosis (Berl) 2018;5:39-40. [Crossref] [PubMed]
- Lippi G, Bovo C, Ciaccio M. Inappropriateness in laboratory medicine: an elephant in the room? Ann Transl Med. 2017;5:82. [Crossref] [PubMed]
- Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7. [Crossref]
- Lippi G, Bovo C, Favaloro EJ. Reflections on the next generation of hemostasis instrumentation. A glimpse into the future? J Lab Med 2016;40:1-7.
- Lippi G, Plebani M, Favaloro EJ. The changing face of hemostasis testing in modern laboratories: consolidation, automation, and beyond. Semin Thromb Hemost 2015;41:294-9. [Crossref] [PubMed]
- Lippi G, Plebani M, Favaloro EJ. Technological advances in the hemostasis laboratory. Semin Thromb Hemost 2014;40:178-85. [Crossref] [PubMed]
- Plebani M, Graziani MS, Tate JR. Harmonization in laboratory medicine: Blowin' in the wind. Clin Chem Lab Med 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. On the complexity of hemostasis and the need for harmonization of test practice. Clin Chem Lab Med 2018; [Epub ahead of print]. [PubMed]
- Favaloro EJ, Jennings I, Olson J, et al. Towards harmonization of external quality assessment/proficiency testing in hemostasis. Clin Chem Lab Med 2018;57:1670-80. [PubMed]
- Favaloro EJ, Gosselin R, Olson J, et al. Recent initiatives in harmonization of hemostasis practice. Clin Chem Lab Med 2018; [Epub ahead of print]. [PubMed]
- Thachil J, Lippi G, Favaloro EJ. D-Dimer Testing: Laboratory Aspects and Current Issues. Methods Mol Biol 2017;1646:91-104. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Recent guidelines and recommendations for laboratory assessment of the direct oral anticoagulants (DOACs): is there consensus? Clin Chem Lab Med 2015;53:185-97. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Reference ranges in hemostasis testing: necessary but imperfect. J Lab Precis Med 2017;2:18. [Crossref]
- Lippi G, Adcock D, Simundic AM, et al. Critical laboratory values in hemostasis: toward consensus. Ann Med 2017;49:455-61. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Preanalytical Issues in Hemostasis and Thrombosis Testing. Methods Mol Biol 2017;1646:29-42. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Post-analytical Issues in Hemostasis and Thrombosis Testing. Methods Mol Biol 2017;1646:545-59. [Crossref] [PubMed]
Cite this article as: Lippi G, Favaloro EJ. Hemostasis practice: state-of-the-art. J Lab Precis Med 2018;3:67.