
The role of the clinical laboratory in the detection and monitoring of acute kidney injury
Introduction
Acute kidney injury (AKI) is a clinical syndrome that is characterised by a rapid deterioration of kidney function that disorganises metabolic, electrolyte and fluid homeostasis over a period of hours to days. AKI complicates the course and worsens the outcome in a significant number of hospitalised patients (1,2). AKI is well recognised as a major health problem that affects millions of patients worldwide. It is often diagnosed in the context of other acute illnesses and is particularly common in critically ill patients (3). The incidence of AKI is 3–20% in hospitalised patients and 30–60% in the intensive care unit (ICU) (4-6). The clinical spectrum of AKI is wide ranging from small subclinical changes in the levels of biochemical markers of kidney function to overt renal failure that requires renal replacement therapy (RRT). The impact and prognosis of AKI can vary considerably depending on the age of the patient, the severity of AKI, the clinical setting, presence of co-morbidities, and also geographical location (4,5,7,8).
Increasing evidence from several studies has been shown the clinical importance of AKI demonstrating that AKI is associated with serious short- and long-term complications (increased mortality and morbidity). Moreover, there is a consistent association with increased long-term risk of poor outcomes such as, progression to chronic kidney disease (CKD) and mortality, longer hospitalisation time and utilisation of health resources (9-15).
The clinical consequences of AKI mainly include the accumulation of waste products, electrolytes, and fluid, but also has less obvious effects, such as reduced immunity and dysfunction of non-renal organs (distant organ cross-talk) (16-18).
AKI is also associated with augmented healthcare costs. The prolonged hospitalisations, in association with the worse outcomes and the diminished quality of life, after discharge, have recognised AKI as a major public health problem (19-21). The reality is even worse if we consider the lack of effective interventions to prevent AKI in patients at-risk and the lack of therapeutic interventions in those affected with AKI.
These observations strengthen the importance:
- To early diagnose AKI in hospitalised patients, in order to implement any available therapeutic intervention to mitigate its impact and
- To identify those hospitalised patients that are at increased risk to develop AKI, in order to prevent its development.
- In order to accomplish this task, we need three things:
- A diagnostic system that will be based in both clinical evaluation and laboratory workup in order to help clinicians to identify patients at risk;
- A clear clinical definition of this syndrome that will help clinicians to determine the cause and stage of AKI and
- Laboratory support that will confirm the diagnosis, help clinicians to stage and monitor the patients during their interventions, and predict the outcome.
- In order to accomplish this task, we need three things:
How the clinical laboratories can support these three demands? In this article, we will review the developments in AKI definition and investigate laboratory contribution to diagnosis and monitoring of patients.
Lost in definitions
The traditional definition
Before 2004 when we used the term acute renal failure (ARF) we tried to define a clinical syndrome that was characterised by a “rapid fall in the rate of glomerular filtration (GFR), which clinically was manifested as an abrupt and sustained increase in the serum levels of urea and creatinine with an associated disruption of salt and water homeostasis” (22). However, this definition had several important limitations that had serious implications for clinical practice.
The terms “rapid”, “abrupt” and “sustained” were not specifically defined. Depending on the cause, ARF can evolve within hours or days. The rapidity of onset may correlate with the severity of the episode, but this is not clear or implied within this definition (23).
There was no precise “biochemical definition” for ARF, which had as a result, mainly in research studies, investigators to use different definitions: some used absolute or percentage increases in creatinine levels, whereas others have used the patient’s need for dialysis. The lack of a precise definition of ARF resulted in more than 30 definitions in the medical literature, which caused wide variation in the reported incidence and clinical significance of ARF. This situation resulted in wide differences on the incidence and outcome, and comparisons between studies was difficult if not impossible (24,25). Although these definitions were developed by researchers in different clinical studies, in clinical practice the situation was much worse. Because of the lack of a uniform definition, each institution has developed its own criteria according to their individual practices. More than 200 different definitions were used in clinical practice as a survey revealed (26). It was clear that a consensus definition was urgently needed to bring order in research and clinical practice. On the other hand, it was realised that the syndrome was much broader and the clinical manifestations, depending on the cause, were ranging from sub-clinical to overt disease. However, these definitions were focusing on the subset of patients with more severe acute kidney disease (AKD) and those with renal failure requiring dialysis treatment. It became clear that a much broader definition was necessary to cover all clinical phenotypes.
Risk, Injury, Failure, Loss, and End-Stage Renal Disease Classification
The term AKI became the preferred term in 2004 when ARF was redefined with the consensus criteria known as RIFLE (an acronym of the Risk-Injury-Failure-Loss-End stage kidney disease) (27). The birth of the RIFLE classification opened a new era for the definition of AKI (Table 1). This was the first consensus definition based on serum creatinine (sCr) levels, estimated GFR and urine output (UO), which provided a platform through which comparative epidemiology could be judged. This classification greatly improved the early detection of AKI.
Table 1
| Stage | Change in serum creatinine | Change in GFR | Urine output |
|---|---|---|---|
| Risk | 1.5-fold increase | Decrease >25% | UO <0.5 mL/kg/h for >6 h |
| Injury | 2.0-fold increase | Decrease >50% | UO <0.5 mL/kg/h for >12 h |
| Failure | 3.0-fold increase or sCr >4.0 mg/dL (350 ìmol/L) with an acute increase of 0.5 mg/dL (44 ìmol/L) | Decrease >75% | UO <0.5 mL/kg/h for >24 h or anuria for 12 h |
| Loss | Loss of kidney function requiring dialysis lasting for >4weeks | ||
| ESRD | Loss of kidney function requiring dialysis lasting for >3 months | ||
ESRD, end-stage renal disease; AKI, acute kidney injury; GFR, glomerular filtration rate; sCr, serum creatinine; UO, urine output.
However, the term AKI has been proposed to encompass the entire spectrum of acute changes in renal function, from minor changes in markers of renal function until the need for RRT. AKI is not synonym to acute tubular necrosis (ATN), nor to ARF. Instead, it encompasses both and also includes other less severe conditions. As a syndrome, it includes patients without actual structural damage to the kidney but with functional impairment relative to physiologic demand. Including such patients in the classification of AKI is clinically attractive because these are precisely the patients who may benefit from early intervention. However, it means that AKI includes both injury and/or impairment.
Rather than focusing exclusively on patients with renal failure or on those who receive dialysis or who have a clinical syndrome defined by tubular necrosis or pathology (which is usually absent anyway), the strong association of AKI with hospital mortality demands to change the way we think about this disorder.
The RIFLE definition was designed to establish the presence or absence of clinical AKI and to describe the severity of this syndrome in a given patient. It did not aim to predict mortality or any adverse outcome (28). However, several studies have shown that there was an association between AKI severity (as described by RIFLE class) with higher mortality and longer hospital and ICU stay (29). The RIFLE criteria have been used in therapeutic trials for AKI, as well as in studies aiming to clarify the pathophysiology of AKI (30,31). Finally, a paediatric version (pRIFLE) proposed in 2007 (32). However, this definition was not perfect. Very soon, Pickering et al. showed that there was a mismatch between increases in sCr concentration and decreases in GFR [estimated with Modification of Diet in Renal Disease (MDRD) or Cockroft-Gault formulae] in the descriptions of risk and failure severity stages (33). A 1.5-fold increase in sCr corresponds to a one-third decrease (not 25%) in GFR and a three-fold increase corresponds to a two-third decrease in GFR (not 75%). If the GFR is not directly measured but estimated by a formula, then results might be also different depending on the formula used. With the MDRD formula a 1.5-fold increase in sCr corresponds to a 37% decrease in GFR, and a three-fold increase in sCr to a 72% decrease in GFR (34).
Acute Kidney Injury Network (AKIN)
In 2007, the AKIN group proposed a modified version of the RIFLE criteria, which aimed to improve the sensitivity of AKI diagnostic criteria for adults (35). There were several changes: the risk, injury, and failure stages became Stages 1, 2, and 3. An absolute increase in sCr of at least 0.3 mg/dL (26.5 µmol/L) within 48 hours was added to Stage 1 which was supposed to make the definition more sensitive; the GFR criterion was removed as a marker of AKI; patients starting RRT were classified as Stage 3, irrespectively of sCr values; and outcome classes were removed. The characteristics of this system are outlined in Table 2.
Table 2
| Stage | Change in serum creatinine | Urine output |
|---|---|---|
| Stage 1 | Absolute increase in sCr >0.3 mg/dL (>26.5 ìmol/L) or > 1.5- to 2.0-fold from baseline | UO <0.5 mL/kg/h for >6 h |
| Stage 2 | Increase in sCr >2.0- to 3.0-fold from baseline | UO <0.5 mL/kg/h for >12 h |
| Stage 3 | Increase in sCr >3-fold from baseline or increase of sCr to >4.0 mg/dL (>354 ìmol/L) with an acute increase of at least 0.5 mg/dL (44 ìmol/L) | UO <0.5 mL/kg/h for >24 h or anuria for 12 h |
sCr, serum creatinine; UO, urine output.
Only one criterion (sCr or UO) has to be fulfilled in order to qualify for a stage. Time becomes more important for AKI diagnosis in the AKIN definition: changes between two sCr values within a 48-hour period are required, while 1 week was proposed by the Acute Dialysis Quality Initiative (ADQI) group in the original RIFLE criteria. Severity of AKI in AKIN is staged over the course of 7 days by the fold-change in sCr from baseline
The AKIN criteria have partially addressed the limitations of the RIFLE system. Although they were not designed to define the cause of AKI, an attempt is made to exclude cases of reversible (or transient) azotaemia or urinary tract obstructions to be characterised as AKI, something that was not possible with the RIFLE criteria (35). However transient azotaemia must be acknowledged as a form of “mild” AKI, since studies have shown it can lead to adverse outcomes (36).
Kidney Disease: Improving Global Outcomes
In 2012, the Acute Kidney Injury Working Group of KDIGO (Kidney Disease: Improving Global Outcomes) revised the AKI definition again (Table 3). This was based on the previous two classifications, and had the aim of unifying the definition of AKI (37). By KDIGO definition, AKI is diagnosed by an absolute increase in sCr, at least 0.3 mg/dL (26.5 µmol/L) within 48 hours or by a 50% increase in sCr from baseline within 7 days, or a urine volume of less than 0.5 mL/kg/h for at least 6 hours. A patient’s progress can be staged over the entire time frame encompassed by an episode of AKI. An increase in sCr up to 3 times from baseline, or a sCr of more than 4.0 mg/dL (354 µmol/L) or initiation of RRT, are all classified as Stage 3.
Table 3
| Definition: AKI is defined as any of the following |
| Increase in sCr >0.3 mg/dL (>26.5 ìmol/L) within 48 hours; or |
| Increase in sCr >1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or |
| Urine volume <0.5 mL/kg/h for 6 hours |
| Staging: AKI is staged for severity according to the following criteria |
| Stage 1: 1.5–1.9 times baseline or >0.3 mg/dL (>26.5 ìmol/L) absolute increase in sCr; urine volume <0.5 mL/kg/h for 6–12 h |
| Stage 2: sCr >2.0–2.9 times baseline; urine volume <0.5 mL/kg/h for >12 h |
| Stage 3: sCr >3.0 times from baseline, or increase in sCr to >4.0 mg/dL (>353.6 ìmol/L), or initiation of renal replacement therapy or, in patients <18 years, decrease in eGFR to <35 mL/min per 1.73 m2; urine volume <0.3 mL/kg/h for >24 h or anuria for >12 h |
sCr, serum creatinine; eGFR, estimated glomerular filtration rate; AKI, acute kidney injury.
KDIGO made one additional change to the criteria: A patient can be classified as Stage 3 due to sCr >4.0 mg/dL (353.6 µmol/L), and in this case it is not required an acute increase of ≥0.5 mg/dL (44.2 µmol/L) to make the diagnosis.
Moreover, it is stated that a rolling baseline can be used over 48-hour and 7-day periods for diagnosis of AKI, while in RIFLE or AKIN it is not clear how this is handled. Finally, changes were also made to severity Stage 3 to enable incorporation of paediatric patients into both definition and staging.
Challenges of applying diagnostic criteria in clinical practice
The issue of time
Time is important in order to define and diagnose AKI. AKI is defined as occurring within 7 days period whereas CKD starts when kidney disease is persisting for more than 3 months. Several studies have shown that some patients may have a slow (but constant) increase of sCr over the course of several days or even weeks and therefore they do not fulfil the consensus criteria for AKI (38,39). The conditions that affect the kidneys can be divided to acute or chronic depending on their duration and whereas CKD is well defined AKI definitions are still evolving. KDIGO in its latest guidelines addressed this issue, introducing the concept of AKD and proposed an operational definition to cover for these cases (37). The AKD definition requires: GFR <60 mL/min/1.73 m2 for <3 months, a decrease in GFR by ≥35%, and an increase in sCr by >50% for <3 months or evidence of structural kidney damage for <3 months. These criteria are currently under revision (2,37).
The issue of baseline renal function
The concept of all AKI definitions is one that requires “a rapid decline in renal function from baseline levels”. This concept is not only needs a definition of a timeframe within which this decline occurs, it also assumes that the patient’s baseline sCr level (which reflects patient’s premorbid kidney function) is also known. This baseline sCr value is necessary to compare with the current value in order to define and stage AKI. Moreover, the baseline renal function is necessary in order to evaluate the extent of renal function recovery after the AKI event, which is a clinically important end point (28). Unfortunately, the baseline sCr level might not be known in a lot of patients, depending on the population studied. Several studies proposed ways to estimate this baseline sCr value in various ways. These included the admission sCr level, the minimum sCr level during hospitalisation, a back-calculation using the MDRD equation, or the lowest value among these. It is a very important decision to be made by the clinician because this is the decision that seriously affects the prevalence, the severity (or staging of patient), and the mortality risk which associated with various stages of AKI.
The admission sCr levels is unlikely to be representative of the true baseline state since it is possible to have been modified by the acute illness that caused the hospitalisation. The lowest sCr level measured during hospitalisation also has a number of disadvantages. First, this measurement is determined too late since the patient’s hospitalisation must have ended in order to identify the nadir value and, therefore, this measurement cannot be used in daily clinical practice and by definition, is a retrospective baseline. Second, the nadir sCr value is likely to be lower or higher than the true baseline level, thereby we may overestimate or underestimate the true incidence of AKI.
When no information on pre-admission renal function is available, for adults, the ADQI has recommended the back-calculation of the baseline sCr value using the MDRD formula, assuming an estimated GFR of 75 mL/min/1.73 m2. Although convenient, the validity of this approximation, as well as that of other surrogate measures of baseline renal function, is questionable and has been the subject of several recent studies and reviews (28,40-44). Some degree of misclassification of AKI exists when someone uses a method to back-calculate the baseline sCr value in both adults and children and this highlights the need to increase efforts to find a true pre-admission sCr measurement that will represent the baseline renal function of the patient (45,46). Looking back into patients’ medical records (when these are available) to obtain a true baseline sCr when the patient was to a stable condition, have been also proposed. There are two ways:
- To use a short time-frame—that is trying to find a measured sCr value within 7 days prior to admission;
- To use a longer time frame—that is trying to find a measured sCr value between 7 and 365 days prior to admission.
KDIGO allows to use the second option since in the general population and in patients with no progressive CKD we can assume that a sCr measurement within the last year and not so close to the event that caused the hospitalisation will reflect their true baseline renal function (28,37,44,47). However, the issue of how to approach a patient when a true baseline sCr value is missing, has not been solved yet, especially when we have to deal with patients at the emergency department. If a patient is admitted with an elevated sCr value, and the true baseline cannot retrieve immediately, a safe diagnosis of AKI can be done only retrospectively. This underlines the serious limitation of sCr as a reliable AKI definition criterion in the acute setting and the need of a more reliable marker that can be used in the acute care setting.
The issue of sCr as a measure of kidney function
Although GFR is considered the best indicator of renal function and its assessment can aid the clinician in estimating the degree of renal dysfunction or the progression of established kidney disease or the estimation of drug dosing, it does not provide information on the cause of kidney disease. It is usually assessed by the renal clearance of a marker that achieves stable plasma concentration, is inert and is freely filtered by the glomerulus but not reabsorbed, secreted or metabolised (48-51). Such an ideal endogenous marker does not exist and for many years sCr has been used as a marker of renal function in both AKI and CKD. Creatinine is a metabolite of creatine. Creatine is a molecule that is synthesised from the amino acids glycine and arginine in liver, pancreas and kidneys and serves as a reserve of high-energy phosphates in skeletal muscle (Figure 1). Creatinine is produced from creatine in the muscles and its production is determined by the amount of creatine generated in liver, pancreas and kidneys, creatine ingested (i.e., intake of red meat) and muscle function. It has a molecular weight of 113 Da, and fulfils most of the requirements for a filtration marker. It is freely filtered by the glomeruli, is not metabolised by the kidney, it is not bound to any protein and it is not toxic (2,52).
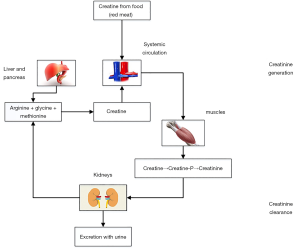
Creatinine is completely cleared by renal excretion when renal function is normal. The proximal tubules also secrete creatinine, which accounts for 10–20% of the excreted load, and this results in overestimation of GFR when measured by creatinine clearance (CrCl) (52-56). When GFR is reduced, the contribution of tubular creatinine secretion increases and may reach 50% of total CrCl, but it is highly variable among individuals. Tubular reabsorption is less important than secretion and appears later in the evolution of the CKD, in patients with already significant alteration in urinary flow in some clinical settings such as decompensated heart failure and uncontrolled diabetes (34,52). Since there is little to no tubular reabsorption of creatinine, its renal clearance is often used to estimate GFR, although its usefulness has been questioned even when the patient is stable (55).
Under stable kidney function, sCr concentration can also reflect skeletal muscle mass if its non-muscle-mass-dependent variations (such as due to renal function or meat intake) can be accurately accounted for. In people with stable kidney function and UO, a 24-h urine creatinine (uCr) is usually a constant number based on skeletal muscle mass and any variation observed is due to changes in meat consumption (57,58). Given the fact that sCr co-varies closely with skeletal muscle mass, its utility in estimating GFR using equations such as MDRD or CKD-EPI may not be appropriate when subjects exhibit weight variations during follow up as in the case of critically ill patients. Muscle loss might be misinterpreted as improvement of renal function (58).
The fact that creatinine is a product of muscle catabolism makes the interpretation of its results problematic in patients with extremely low or high muscular mass (59). This may explain why the same sCr value correspond to different GFR in subjects of different age, sex and ethnicity (59).
In health, it is produced at a constant rate and the rate of production is matched by the rate of renal excretion. However, large and sustained falls in production have been demonstrated during critical illness. A true fall in GFR may not be adequately reflected by sCr in patients with sepsis, liver disease, and/or muscle wasting (60,61).
Creatinine-based criteria for AKI often do not take into account underlying renal reserve. Up to 50% of the kidney function maybe lost before we see any detectable rise in sCr. The role of creatinine as a marker of renal function is limited by the fact that its half-life increases from approximately 4 h to 24–72 h if the GFR decreases, depending on the degree of decrease (62). As such, the serum concentration may take 24–36 h to rise after a definite renal insult (2).
SCr concentrations are also affected by drugs, which compete with tubular secretion. In this case, sCr levels may fluctuate without a change in renal function (2,59,63).
The measurement of sCr in the clinical lab is performed either by enzymatic or the Jaffe methods. Both are colorimetric and although the enzymatic methods are exhibiting better specificity and sensitivity both methods are not fully specific for the measurement of creatinine (59). No method is free from interferences and substances like bilirubin or drugs may interfere with certain analytical techniques, more commonly with Jaffe-based assays. Harmonisation of sCr measurement between laboratories is important, especially when a physician is seeking past measurements from patients’ medical records that may have been performed in a different laboratory and with a different commercial assay and wants to compare with the current value. Theoretically standardisation of sCr measurements has been achieved since the Creatinine Standardisation Program developed a reference method based on isotope dilution mass spectrometry (IDMS) and it has been requested that all manufacturers standardise their commercial kits to this IDMS reference procedure and their calibrators to be traceable to a higher-order method by 2007 (59,64,65). However, several studies since then have shown that several commercial methods still provide results that are deviate significantly from the true value as this is determined by the IDMS reference method. The enzymatic assays seem to perform better in aspect and exhibit less inter-assay variability compared to Jaffe assays (66-68). Enzymatic assays seem also to perform better than Jaffe assays in terms of precision (64,69).
sCr is measured as a concentration and is therefore affected by variations in volume status. Aggressive fluid administration may dilute creatinine in blood. Studies have shown the effect of fluid accumulation on sCr concentration. Increase of total body water dilutes sCr altering the volume distribution resulting in overestimation of kidney function, and underestimation of AKI severity. Moreover, the diagnosis of AKI may be delayed or missed in patients with significant fluid shifts or fluid overload (70-72). A recent study revealed that AKI was diagnosed or classified differently in up to 18% of patients after sCr levels were adjusted for net fluid balance and estimated total body water. Affected patients had mortality rates similar to those with AKI that was present before adjustment. The researchers suggested the use of an adjusted creatinine on the basis of fluid balance (71). More recently Pickering et al. proposed a model that combined volume and creatinine kinetics to assess changes in renal function. This model also takes into account fluid type, the rate of fluid infusion and urine output (73).
The issue of small changes in sCr
Current consensus definitions require small changes in sCr (26.5 µmol/L or 0.3 mg/dL or 50% from baseline to peak) for diagnosis of AKI. These definitions do not take into account the magnitude of baseline creatinine value, the intra-individual biological variation (CVi) of sCr and the numerous factors that interfere with its laboratory measurement (44,59).
In a patient with normal kidney function (i.e., sCr levels 0.70 mg/dL), a rise by 0.3 mg/dL may indeed be due to an important reduction in GFR. In contrast, in patients with underlying CKD, (i.e., baseline sCr levels 3.0 mg/dL) a rise by 0.3 mg/dL may be within the acceptable daily variation and simply reflect an inconsequential change in GFR. This is particularly relevant when diagnosing AKI Stage 3 using KDIGO guidelines, defined by a rise in sCr to >4.0 mg/dL (≥353.6 µmol/L). A patient with a baseline sCr of 3.9 mg/dL (345 µmol/L) whose sCr rises by 0.3 mg/dL in 48 h would be classified as having KDIGO AKI Stage 3, whereas the same absolute rise would be defined as AKI Stage 1 in a patient with normal baseline renal function (44,74-76).
In the literature, there are studies that demonstrate that even smaller changes (i.e., 8.8 or 0.1 mg/dL or 1–24%, baseline to peak) in patients, are independently associated with a 45% increase of end-stage renal disease (ESRD) or a two-fold risk for CKD (77-81). These associations of such small changes with adverse outcome, in published studies, are questionable and may reveal the ability of confounders and not AKI to influence the development of CKD.
Moreover, there are questions beyond the association of small changes and outcome. The first question is how these minimal changes are defined. In most cases the definition is arbitrary and not uniform across all studies. Another important question is whether these changes represent true changes in a patient’s health status or just random variation. The answer is not so straightforward. These studies do not take into account the variability in sCr measurement and its relevance to AKI. These small changes in sCr do not always reflect true changes in renal function as may be within the limits of the combined analytical and biological variation (BV). It depends also on the baseline sCr of a specific patient. In order to estimate if a change in sCr represents a true change in patients’ health status and it is not just a random variation we must take into account not only the base line value but also the analytical and BV, the major sources of variation in laboratory results (43,44,59). For every analyte we measure in the lab there is a physiological random variation around a homeostatic set point that can be measured and is expressed as a coefficient of variation (CV). This homeostatic set point and CV is different for each individual and is termed within subject or CVi. Briefly, CVi can be calculated from serial measurements in a number of stable patients, under the same conditions, on a relatively short period of time. This variation is independent from analytical variation, cannot reduced and should be taken into account together with analytical variation once we try to determine when two consecutive measurements, of the same analyte in a patient, differ and this difference is of clinical significance. A part of BV of creatinine could be explained from to day-to-day variations in true GFR.
We can calculate objectively the true change is sCr that represents a true change in patients’ health status by calculating the reference change value (RCV) using the following formula: RCV = 21/2 * Z * (CVA2 + CVI2)1/2 where, CVA = analytical CV, CVI = within subject BV and when Z =1.96, then a change in any direction (two-tailed) to the RCV is “significant” with 95% probability (82).
BV can also show the way to the clinical laboratory for optimal analytical performance based on objective criteria (83). An optimal CVA is considered the half of the CVI. The CVI for sCr (can be found in the literature) is 5.9% for healthy people and it is the median from 28 published studies. Therefore, an optimal performance for sCr requires CVA ≤3.0. Enzymatic methods for sCr measurement are exhibiting lower CVA compared to Jaffe methods (5.5% vs. 2.0%) and fulfil better the quality criteria (59,64,69). This means that depending on the method used by the lab significant changes in sCr can be either 19% (Jaffe methods) or 13% (enzymatic methods).
This approach has several implications for research and clinical practice:
- The clinician must know the lab’s method that sCr was measured;
- In a clinical practice, as in research practice, we cannot combine results from different labs even when they perform the same method since CVA may differ;
- The fixed value (0.3 mg/dL), that the current criteria use to define AKI, may need a reconsideration. Relative increases from baseline for each patient that take into account the analytical and BV maybe should be incorporated into AKI definition (84,85).
Several examples in the literature have shown that if we apply the fixed KDIGO’s criteria in the definition of AKI, may be underestimated if the patient (adult or child) has a baseline value at the low end of normal range (i.e., 0.7 mg/dL) and on the other hand may be overestimated if the patient is a CKD with a baseline 3.0 mg/dL (43,44,59).
Some restrictions on the RCV calculation must be noted here. It is well known that BV is not the same in health and disease. Patients with chronic conditions (such as CKD, liver disease or diabetes) might exhibit higher BV than healthy subjects (86-91). Therefore, the baseline renal function as well as chronic conditions should be taken into account (when these are known) and the appropriate BVI incorporated into calculations (90). The estimates of BV were derived from healthy subjects or stable CKD patients under highly standardised conditions, in the absence of factors that interfere with assay specificity. In acutely ill people BV is higher and therefore higher RCV values might be needed in order to determine that a change is clinically significant. Therefore, the use of BV derived from healthy people may underestimate the true RCV in patients with acute conditions (as those with AKI). However, in such conditions it is difficult to estimate how much of the observed variation is random. In consequence the use of healthy population RCVs might lead to labelling many hospitalised patients’ test results as having changed significantly. Such changes could be called “false positives” since real RCV would be higher than in the healthy population. On the other hand, the RCV published in the literature, uses the assumption that the values found in the studied subjects are forming a Gaussian distribution. This assumes truly random variation and also means that there is no correlation between successive results (82,92). This seems to be reasonable when we perform tests at medium to long-term intervals between samples. However, when we perform tests more frequently (on a daily basis), this serial correlation might exist. Estimates of within subject BV over short periods of time might be smaller than long-term estimates. This auto-correlation could make the CVi and therefore RCV smaller. Therefore, the RCV that uses CVi from healthy people will lead to “false negative” changes. However, these two effects tend to balance each other out and thus calculating RCV from healthy subjects is valid and widely applicable (82). The estimation of RCV has generated debate and discussion regarding the statistical approach that should be applied, especially when the analyte under investigation does not exhibit a normal distribution. When the analytes exhibit normal distribution, the standard approach proposed by Fraser can be applied for the determination of RCV. On the other hand, a non-parametric approach might be more appropriate for the determination of RCV in analytes that do not follow the normal distribution and are highly skewed. However, the different approaches that have been proposed need careful validation (93-98).
And finally, despite the significant amount of work relating to BV over the last 50 years, the published papers are of varying quality in terms of study designs and presentation as well as the use of non-standardised terminology to describe the data. This delivers a high degree of uncertainty around published estimates of BV (99-101). It is well recognised that there is a need to further develop criteria to better characterise BV data and this work has been undertaken by the Biological Variation Working Group (BVWG) established by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) (90,102-104).
The issue of UO
UO is a rapid bedside test for renal function and oliguria has been one of the oldest biomarkers for renal injury (105). It can be measured in real time, it is easy to measure and inexpensive. It is an important clinical marker since urine flow variations trigger first attempts to therapy but, like creatinine, it is not renal specific (106,107).
Although the relation of oliguria with ARF has been made more than 200 years ago, its systematic inclusion in the definition of AKI occurs with the adoption of the RIFLE criteria as an alternative to sCr criteria. It remained unchanged in the AKIN and KDIGO criteria. UO criteria and sCr elevations have been considered of equal importance in all AKI consensus definitions (105).
The theoretical advantages of UO over sCr include:
- The speed of the response. A rapid reduction of UO may be the earliest indication of decreased kidney function. For example, if GFR were to suddenly fall to zero, a rise in sCr would not be detectable for several hours. On the other hand, UO would be affected immediately;
- Low UO is defined by a predefined cut-off value. There is no need to look for a baseline UO. In contrast, sCr based definitions depend on a baseline sCr value which is often unknown and has to be estimated by processes that introduce significant errors;
- Certain conditions (infections, sepsis, malnutrition) seriously affect creatinine production and make sCr use an unreliable surrogate marker of GFR.
Oliguria is a complex process. There are multiple mechanisms that can potentially cause oliguria in AKI, therefore it is not highly specific or sensitive marker of parenchymal ischaemic injury (108). These mechanisms include overall reduction or regional intra-renal differences in blood flow and redistribution, glomerular injury, altered intra-glomerular haemodynamics, impaired tissue oxygenation causing preferential ischaemia to the S3 segment of the proximal convoluted tubule and the oxygen-avid thick ascending loop of Henle, loss of osmolar gradient, interstitial oedema or inflammation and finally tubular or lower urinary tract obstruction (108). The decline of GFR and UO in response to a decrease of renal blood flow (RBF) is classically referred to as pre-renal azotaemia, which can evolve into structural damage if renal hypoperfusion persists. Into this context UO is used not only as a marker of AKI, but also to guide fluid resuscitation in critically ill patients. The mechanisms of diuresis regulation are discussed in two excellent reviews (108,109). Oliguria does not occur at the same time with sCr changes, so this criterion might be used to identify AKI earlier or may also contribute to select different patients from those selected by sCr criteria (110,111).
However, the major difficulty to measure UO over a period of time of 6 to 12 hours is that can be measured accurately only in patients with a urine catheter. Another problem of UO, as a biomarker, is that may change in conditions not related to AKI:
- As a physiological response (reflects conditions associated with antidiuresis related to hypovolaemia);
- As an indicator of stress (oliguria may occur due to pain, surgery trauma) and finally
- As an indicator of failing glomerular flirtation.
It is well recognised that hydration status, use of diuretics and haemodynamic status influence UO in the absence of AKI. On the other hand, it is also known that severe AKI can occur with normal UO. However, the ADQI group has decided in the RIFLE consensus definition to use UO criteria to define and stage AKI which remained and in the subsequent definitions (AKIN, KDIGO) (27,35,37). The accuracy and the usefulness of this criterion in clinical practice are not well verified. The measurement of UO has to be done manually and inputted into the hospital’s information system, which renders it to clerical errors. There are difficulties in measuring, monitoring and recording accurately UO. Therefore, it is often omitted as criterion from clinical studies (29).
The UO criterion has been assessed in several studies, where mostly critically ill patients are involved. However, the number of studies is relatively small compared to those that use sCr criteria (106,107,110,112-116).
The issue of GFR measurement in AKI
GFR, which measures the amount of plasma filtered through glomeruli within a given period of time, is a physiologic process and as such a direct indicator of kidney function. It is well known that the reduction of the GFR, secondary to either AKI or CKD, are accompanied by increases in sCr. However, sCr is an insensitive surrogate biomarker in the measurement of GFR, since its increase does not parallel the fall of GFR, in a timeframe that is clinically useful. The insensitivity of this surrogate marker as a measure of GFR is not uniformly appreciated. Neither sCr nor one of several derived equations to estimate GFR (eGFR), based primarily on the sCr, can be used in AKI, nor can they be used reliably over the entire range of GFR to estimate it safely (59,76).
In higher GFRs very large changes in GFR are needed to result in small changes in sCr and the opposite is true for lower GFRs. In addition, the formulas that use a single measurement of sCr to estimate GFR, were derived in patients with CKD and rely on the assumption that the patient is in-steady state and creatinine production and excretion remains constant, which is not the case in AKI patients where changes in sCr are usually delayed and follow GFR changes. On the other hand, during the recovery phase the improvement of GFR usually precedes the sCr decline by several days. These problems were highlighted in a recent, excellent article that documents the need for a true GFR measurement in patients with AKI (76). Moreover, mathematical models have been proposed to predict GFR on the basis of sCr changes during AKI but do not seem to be practical for routine applications (70). Jelliffe et al. developed an equation, that has been validated recently, to estimate GFR in the setting of non-steady kidney function (117,118). Other methods that have been proposed include continuous monitoring of the GFR for ICU patients and short time urine collections (2–8 h) with a blood sample for CrCl determination (119,120).
On the other hand, we can measure GFR with a direct method. The gold standard for GFR determination, is the renal clearance of inulin. However, it is rarely performed due to inconvenience and high cost. Today a number of filtration markers and several protocols have been proposed to replace it (121). Of note here is that the proposed protocols do not include only urinary clearance but plasmatic clearance has also proposed. Iohexol, a non-ionic contrast agent, is most suited to replace inulin as the marker of choice for GFR determination. Iohexol fulfils all requirements for an ideal GFR marker (low extra-renal excretion, low protein binding, it is secreted nor reabsorbed by the kidney). In addition, iohexol is virtually non-toxic and carries a reasonable cost. Moreover, as iohexol is stable in plasma, administration and sample analysis can be separated in both space and time, allowing access to GFR determination across different settings. Iohexol can be measured by high performance liquid chromatography with ultraviolet detection (HPLC-UV), X-ray fluorescence (XRF) and liquid chromatography-tandem mass spectrometry (LC-MS/MS), with HPLC-UV to be the most commonly used method in Europe. This method is sensitive, specific and reproducible, enabling the use of very low doses of iohexol, since it presents with very low limits of detection. Additionally, an international external proficiency program (operated by Equalis AB in Sweden), allows the inter-laboratory comparison of results. This method can be easily adopted in most modern clinical laboratories today. Plasma clearance measurement is the protocol of choice as it combines a reliable GFR determination with convenience for the patient. Single-sample protocols dominate, but multiple-sample protocols may be more accurate in specific situations (122,123). However, the methods of iohexol administration (single bolus or continuous infusion) is a matter of debate especially for patients with AKI (124,125).
Complementary tools to aid AKI diagnosis and management
In certain clinical circumstances, it is necessary to use additional tools to diagnose AKI, especially in clinical cases where sCr and UO, don not change significantly, are misleading, or cannot be interpreted accurately. This is particularly relevant for ICU patients in whom critical illness is usually accompanied by the presence of fluid overload, muscle wasting, sepsis, and reduced effective circulating volume all of which may completely mask the diagnosis of AKI. Several research groups have proposed that novel biomarkers could be used to define and stage AKI in conjunction with RIFLE or AKIN criteria. A meta-analysis of data from 19 studies conducted in eight countries, involving 2,538 patients, of whom 487 (19.2%) developed AKI, reported that neutrophil gelatinase-associated lipocalin (NGAL) levels in plasma, serum or urine seem to be of diagnostic and prognostic value for AKI, RRT and mortality, especially in patients who have undergone cardiac surgery, as well as in children population. NGAL can allows the early diagnosis of AKI in a few hours, after the onset of kidney damage with increased specificity and sensitivity (126).
Urinary electrolytes
For many years the measurement of urine electrolytes was a useful tool in AKI management. Its main utility was to distinguish a functional renal impairment (“pre-renal AKI” or “pre-renal azotaemia”), generally associated with low renal perfusion, and a structural renal impairment (“renal AKI” or “intrinsic AKI”), in which there is tubular damage leading to an inability to properly reabsorb electrolytes, including sodium.
In situations associated with transient hypovolaemia or hypoperfusion, healthy kidneys respond by increasing urine osmolarity and reducing sodium and/or urea or uric acid excretion. However, this physiological response may be variable and confounded by CKD and various medical interventions, such as diuretic therapy, use of antibiotics (aminoglycosides), and cardiopulmonary bypass (127-129). Whereas the presence of low fractional excretion of sodium (<1%), uric acid (<12%), and urea (<35%) together with a normal urinary sediment may support the diagnosis of functional AKI, the absence of these typical urinary electrolyte abnormalities would not exclude it (Table 4) (130,131). Finally, low fractional excretion of sodium (FENa) values have also been observed in experimental sepsis with increased RBF as well as in the first hours of sepsis in humans (115,132,133). Moreover, urinary electrolytes and FENa, fractional excretion of urea (FEUrea), or uric acid (FEUA) has not been consistently shown to have clear correlations with clinical and histopathological findings (134-136). As such, the interpretation of urinary electrolytes is challenging especially in the critical care setting (137). A single measurement of urinary electrolytes has a limited role in determining the differential diagnosis of AKI in critically ill patients. Instead, serial monitoring of urinary electrolytes may be more useful as sequential alterations in urine composition have been shown to parallel the development and severity of AKI (138-140). However, whether serial measurement of urine electrolytes can also help diagnosing the aetiology of AKI remains unclear.
Table 4
| Test | Pre-renal azotemia | Intrinsic AKI | Post-renal obstruction |
|---|---|---|---|
| Urine Na (mmol/L) | <20 | >40 | >40 |
| FENa | <1% | >2% | Variable |
| FEUrea | <35% | >35% | – |
| Urine/serum creatinine | >40 | <20 | <20 |
| Specific gravity | >1.020 | 1.008–1.012 | ~1.010 |
| Osmolality (mOsm/KgrH2O) | >500 | <300 (near serum) | <500 |
| Urine/serum osmolality | >1.5 | <1.3 | <1.5 |
AKI, acute kidney injury.
Measurement of urinary Sodium in 24-hour collections
Twenty-four-hour urine collections are regarded as the “gold standard” to measure sodium urinary excretion and are useful to estimate daily dietary salt intake particularly in the management of patients with hypertension. However, this method have problems mainly due to the difficulties associated with the accurate collection of a complete 24-h collection (141-143). In hospitalised patients and especially in critically care setting disturbances in fluid and electrolytes are the most common problems and are associated with increased morbidity and mortality (144,145). Severe burns, trauma, brain trauma and heart failure can lead to disturbances in fluid and electrolyte homeostasis. Reduced perfusion to the kidney (due to hypovolaemia or hypotension), activation of hormonal systems (renin-angiotensin-aldosterone and vasopressin), tubular damage (caused by ischaemia or nephrotoxic drugs) and inappropriate use of fluids and electrolyte solutions are the most common causes. Hypernatraemia is very common and important electrolyte disorder in critically ill patients and is related to increased administration of solutions containing sodium, renal water loss (i.e., use of diuretics), and decreased capacity to excrete sodium especially in the setting of AKI (137,144,146). It seems that 24-hour urine collections have a role in calculation of total electrolyte excretion and may be helpful to prevent the effect of electrolyte overload in multiple organs (147). Moreover, hypernatraemia is often thought to be hypovolaemic since it is associated with increased water loss. However, hypervolaemic hypernatraemia has been described in ICU patients that are recovering from AKI, a condition that is characterised by massive retention of total body sodium and total body water. Recent studies have shown that this condition might not be so rare as it was initially thought and measurement of electrolytes in urine may be helpful in diagnosis and management (148-150).
FENa
The calculation of the fraction of a urine solute that is excreted compared to the amount that is filtered is a concept that was developed in the early 1970s. During this decade, FENa was developed for use as diagnostic tool to distinguish pre-renal azotaemia from ATN. However, the studies that proposed urinary Sodium and FENa as a useful tool for this distinction are not only several decades old but he cut-off they proposed were derived from a small number of patients with very increased serum urea and creatinine suggesting that only patients with severe AKI were included. Moreover, patients receiving diuretics or were non-oliguric were excluded (151,152). FENa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the sodium filtration rate to the overall GFR rate (estimated by the renal filtration of creatinine). A euvolaemic person with normal renal function and moderate salt intake in a steady state will have FENa approximately 1%. When interpreting FENa it is necessary to consider whether the patient has pre-existing CKD as these patients might exhibit FENa >1% in the absence of AKI depending on their GFR and daily dietary sodium intake (153,154). In a case of pre-renal azotaemia the epithelial cells of proximal tubules reabsorb filtered sodium resulting in a very low concentration of sodium in urine (<20 mmol/L) and FENa <1%, whereas in established AKI concentration of sodium in urine is higher than 40 mmol/L and the resulting FENa is >1%. A low FENa or low urine sodium reflects poor renal perfusion of any cause, not exclusively volume depletion. However, there are many causes for a low FENa despite AKI and for a high FENa despite pre-renal AKI. The use of diuretic agents, the presence of sepsis, myoglobinuria, acute glomerulonephritis, cirrhosis, congestive heart failure, and contrast induced nephropathy may seriously affect the performance of this test (127,155-160). A detailed list of the limitations of this test is presented in the articles Perazella et al. and Diskin et al. (128,161).
Fractional excretion of urea (FEUrea)
The calculation of FEUrea is based on the same principle as FENa. Urea conservation accompanies water conservation and it has been shown that its reabsorption occurs mainly (57%) at the proximal segment of the nephron, it is practically not affected by diuretic agents, which act distally to the proximal tubule (162,163). Therefore, it should be more reliable than FENa. However, studies that evaluated the performance of FEUrea in various clinical settings (including ICU patients) have produced discordant results (127,164-167). A low FEUrea is usually indicative of pre-renal AKI, however recent studies indicate that ageing, sex certain drugs and sepsis may alter FEUrea (128,159,168). This is understandable since urea movement across membranes is modulated by specific urea transporters, drugs or disease entities may interfere with urea’s active transport and therefore alter FEUrea (128).
How can the clinical lab be of help?
It is easy to implement these calculated tests in a clinical lab’s routine. A fresh random urine collection (not catheterised) is required, together with a blood sample, and calculations can be made within the laboratory information system (LIS). However, the utility of standardised interpretive comments for these tests are a matter of debate. Personalised interpretive comments can be made by the Clinical Chemist only if he/she has access to patients’ history and putative diagnosis, and may be limited by regulatory restrictions.
In conclusion the interpretation of urinary electrolytes is challenging, with many limitations affecting urine concentrations and fractional excretion indices. Serial monitoring of urinary electrolytes may be more useful than individual measurements, as sequential alterations in urine composition have been shown to parallel the development and severity of AKI. However, whether serial measurement of urine electrolytes can also help diagnosing the aetiology of AKI remains unclear
Urine microscopy (UM)
UM is an important tool for the diagnosis and management of several pathological conditions that affect the kidneys. Examination of urinary sediment is one of the oldest tests used to evaluate AKI in clinical nephrology (152,169,170). Evaluation of urine sediment is often considered as a complementary measure for the diagnosis and the severity of AKI since it can provide additional information (136,171). UM has many advantages: it is cheap, non-invasive and readily available. Traditionally urinalysis is a manual method that includes visual inspection of urine, chemical analysis and microscopic analysis of the sediment. There is no reference method for urine sediment microscopy. Manual urine sediment analysis is still the gold standard in the laboratory. In most laboratories a bright field microscopy of unstained native urine is the mainstream urine examination (172,173).
Recent technological advances have led to the production of automated instruments based on flow cytometry or digitised microscopy and are currently available for routine use in large clinical laboratories. These tools allow the examination of large numbers of samples in a short period of time. One major advantage of these instruments is that the actual images of selected urine samples can be stored in a computer and transmitted easily to nephrologist for clinical evaluation (174).
However, preanalytical protocols still vary between laboratories. The preanalytical phase is the most important and the most vulnerable part of urinalysis. It accounts for no less than 75% of all laboratory errors. In an effort to standardise urinalysis EFLM (European Federation for Laboratory Medicine) has produced a European guideline which provide specific instructions for urinary sediment analysis. UM can provide very valuable information when performed by a skilled operator, using a freshly collected non-catheterised urine sample (175).
When performed properly, the presence and the type of casts in urine sediment can differentiate the aetiology of AKI. Visualisation of red cell casts is due to glomerulonephritis, whereas the presence of renal tubular epithelial cells and coarse granular or muddy brown casts as well as casts containing tubular epithelial cells is indicative of ATN (Table 5) (176). On the other hand, absent sediment or the presence of occasional hyaline casts is indicative of pre-renal azotaemia (152,169,170,177). An ischaemic or nephrotoxic insult causes tubular injury, which results in apoptosis or necrosis of the renal tubular epithelial cells. These are shed into the tubular lumen where they are excreted free or form casts which can be examined in fresh urine sediments. Since pre-renal azotaemia and AKI are not separate clinical entities but rather are a continuum, the presence of cells and casts would be expected to increase with the severity of the disease. It is logical to try to assess these findings quantitatively (178,179). However, evidence that establishes the diagnostic value of UM has largely been lacking. Two systematic reviews evaluated the usefulness of urinary microscopy and suggested that it may have a limited role for the diagnosis, classification and prognosis of septic AKI (134,135). However recent data show that UM may have a complementary role for the discrimination of septic from non-septic AKI (180). Another systematic review evaluated the usefulness of urinary microscopy for the differentiation between pre-renal AKI and ATN and suggested that its clinical utility may be increased by the use of a simple urinary scoring system, based on the number of renal tubular epithelial cells and casts (181).
Table 5
| Urine sediment | Finding | Suggestive of |
|---|---|---|
| Cells | Squamous epithelial cells | Normal |
| Renal tubular epithelial cells | Acute tubular injury | |
| Red cells (non-dysmorphic) | Bleeding that can be anywhere in the urinary tract (not glomerular bleeding) | |
| Dysmorphic red cells | Glomerular disease (if the urine sample is not fresh) | |
| White blood cells | Normal if <3 per high power field | |
| >3 urinary tract infection, pyelonephritis, interstitial nephritis | ||
| Casts | Red cell casts | Diagnostic of glomerular disease (glomerulonephritis, lupus nephritis, vasculitis) |
| White blood cell casts | Renal Infection (pyelonephritis, interstitial nephritis) | |
| Hyaline casts | Normal or pre-renal disease | |
| Granular casts and/or muddy brown casts | Tubular necrosis (muddy brown casts contain necrotic tubular epithelia cells) | |
| Crystals | Urate/phosphate | Not specific finding of acute kidney injury |
| Healthy individual may have some crystals in urine | ||
| Abnormal crystal presentation in patients’ urine may be indicative of metabolic disorders, due to medications, or indicative of postrenal obstruction | ||
| Microorganisms | Bacteria | Indicative of urinary tract infection but can be present due to sample contamination |
In conclusion examination of urinary sediment can be helpful in differentiation of prerenal AKI from ATN, may have a role in determining the severity of AKI and can be more specific than some novel biomarkers in early detection of AKI, although lacks sensitivity (131,179,182).
However, we must point out that the clinical utility of UM may be limited for several reasons. First apart of renal biopsy, there is no gold standard that would be able to diagnose AKI in a given patient, therefore we cannot judge the performance of any biomarker objectively and its usefulness is likely to remain controversial. Second interpretation of UM is highly dependent on the training and experience of the user, and the clinical lab should guarantee its competency in the preparation and interpretation of UM (183,184). Third manual microscopy, if performed by an experienced clinical chemist or nephrologist, outperforms automated urinalysis systems mainly because the latter uses non-centrifuged urine, and should be preferred (185-187). Centrifugation increases the probability of locating casts. Finally, the development of a standardised and validated scoring system based on the number of tubular epithelial cells and renal casts is necessary (181).
Generating e-alerts for AKI. The contribution of the clinical laboratory
The term “e-alert” has been used widely the last few years in the research setting of AKI. It is an area that the clinical laboratory can contribute not only by the laboratory results but also has the infrastructure (LIS) that is required to implement these e-alerts. We believe that it worth discuss in brief first what these e-alerts are and second to look at the recent research developments in this fast-evolving area.
“e-alerts” or “early warning systems” are intended to enable earlier detection of AKI and comprise of two essential components. The first component is the detection of AKI, which is a rule-based or mathematical process that requires the creation of an algorithm to compare a patient’s current sCr levels with a previous one using the current internationally accepted diagnostic criteria and can be incorporated into laboratory’s LIS. Wherever possible, sCr measurements are considered before inpatient admission and patients needing chronic dialysis are excluded. The same principle can be applied to UO data. Theoretically and depending on the AKI definition that will be used and the algorithm we can achieve diagnosis and staging of a patient. The second component is the “alerting process” and this has to do with how these changes are communicated to the patient’s physician. During the alerting process, treating physicians can be informed about the reduction in renal function in various ways. One way can be just a simple list of affected patients with or without mention of their AKI’s severity grade. Another way is by using technically sophisticated early warning systems that will alert doctors with a message. This can be linked to recommendations to treating physicians (188-190).
The drivers behind this concept were the recognition that routine clinical practice often was not fast enough to diagnose AKI timely, especially on weekends and most often patients were managed by non-specialist nephrologists (191-194).
Conclusions
A universally accepted definition of AKI is necessary for its diagnosis and management. Current developments to standardise the definition of AKI are significant and despite the uncertainties that still exist in several areas, have helped in both clinical management and research. However, these definitions which are based on sCr changes and UO do not allow a biochemical definition of this syndrome and clinical judgement is necessary. These definitions are designed not to replace clinical diagnosis but rather to complement or assist it. The contribution of the clinical lab is hugely important not only to help clinicians interpret correctly these changes but also to highlight their limitations. Evolution of AKI definitions should take these concerns into account. The contribution of the lab emerges also in new areas as in the creation of electronic alerts as it has the infrastructure (LIS) to implement them.
Acknowledgments
I would like to thank Dr. Loukia Spanou, MD. for her valuable comments during the preparation of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Pierre Delanaye) for the series “Nephrology and clinical chemistry” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.07.06). The series “Nephrology and clinical chemistry” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Makris K, Spanou L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin Biochem Rev 2016;37:85-98. [PubMed]
- Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care 2016;20:299. [Crossref] [PubMed]
- Bellomo R, Ronco C, Mehta RL, et al. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care 2017;7:49. [Crossref] [PubMed]
- Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet 2013;382:170-9. [Crossref] [PubMed]
- Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014;10:193-207. [Crossref] [PubMed]
- Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411-23. [Crossref] [PubMed]
- Olowu WA, Niang A, Osafo C, et al. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health 2016;4:e242-50. [Crossref] [PubMed]
- Abd ElHafeez S, Tripepi G, Quinn R, et al. Risk, Predictors, and Outcomes of Acute Kidney Injury in Patients Admitted to Intensive Care Units in Egypt. Sci Rep 2017;7:17163. [Crossref] [PubMed]
- Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365-70. [Crossref] [PubMed]
- Thakar CV, Christianson A, Himmelfarb J, et al. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 2011;6:2567-72. [Crossref] [PubMed]
- An HJ, Yu CS, Yun SC, et al. Adjuvant chemotherapy with or without pelvic radiotherapy after simultaneous surgical resection of rectal cancer with liver metastases: analysis of prognosis and patterns of recurrence. Int J Radiat Oncol Biol Phys 2012;84:73-80. [Crossref] [PubMed]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012;380:756-66. [Crossref] [PubMed]
- Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 2012;81:819-25. [Crossref] [PubMed]
- Perinel S, Vincent F, Lautrette A, et al. Transient and Persistent Acute Kidney Injury and the Risk of Hospital Mortality in Critically Ill Patients: Results of a Multicenter Cohort Study. Crit Care Med 2015;43:e269-75. [Crossref] [PubMed]
- Adams HH, Hibar DP, Chouraki V, et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat Neurosci 2016;19:1569-82. [Crossref] [PubMed]
- White LE, Hassoun HT. Inflammatory Mechanisms of Organ Crosstalk during Ischemic Acute Kidney Injury. Int J Nephrol 2012;2012:505197 [Crossref] [PubMed]
- Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology 2012;116:1139-48. [Crossref] [PubMed]
- Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int 2016;89:555-64. [Crossref] [PubMed]
- Collister D, Pannu N, Ye F, et al. Health Care Costs Associated with AKI. Clin J Am Soc Nephrol 2017;12:1733-43. [Crossref] [PubMed]
- Silver SA, Chertow GM. The Economic Consequences of Acute Kidney Injury. Nephron 2017;137:297-301. [Crossref] [PubMed]
- Silver SA, Long J, Zheng Y, et al. Cost of Acute Kidney Injury in Hospitalized Patients. J Hosp Med 2017;12:70-6. [Crossref] [PubMed]
- Lameire NH, Vanholder RC. Acute renal failure: pathophysiology and prevention. In: Davison AM, Cameron JS, Grunfeld JP, et al. editors. Oxford textbook of clinical nephrology. 3rd edition. Oxford (UK): Oxford University Press, 2005:1445-64.
- Kellum JA, Levin N, Bouman C, et al. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care 2002;8:509-14. [Crossref] [PubMed]
- Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med 2008;36:S152-8. [Crossref] [PubMed]
- Hilton R. Defining acute renal failure. CMAJ 2011;183:1167-9. [Crossref] [PubMed]
- Ricci Z, Ronco C, D'Amico G, et al. Practice patterns in the management of acute renal failure in the critically ill patient: an international survey. Nephrol Dial Transplant 2006;21:690-6. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol 2011;7:201-8. [Crossref] [PubMed]
- Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 2008;73:538-46. [Crossref] [PubMed]
- Bagshaw SM, Gibney RT, McAlister FA, et al. The SPARK Study: a phase II randomized blinded controlled trial of the effect of furosemide in critically ill patients with early acute kidney injury. Trials 2010;11:50. [Crossref] [PubMed]
- Lentini P, de Cal M, Cruz D, et al. The role of advanced oxidation protein products in intensive care unit patients with acute kidney injury. J Crit Care 2010;25:605-9. [Crossref] [PubMed]
- Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007;71:1028-35. [Crossref] [PubMed]
- Pickering JW, Endre ZH. GFR shot by RIFLE: errors in staging acute kidney injury. Lancet 2009;373:1318-9. [Crossref] [PubMed]
- Endre ZH, Pickering JW, Walker RJ. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI). Am J Physiol Renal Physiol 2011;301:F697-707. [Crossref] [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [Crossref] [PubMed]
- Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant 2010;25:1833-9. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group-KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int 2012;Sup 2:1-138.
- Ostermann M, Chang RW. Challenges of defining acute kidney injury. QJM 2011;104:237-43. [Crossref] [PubMed]
- Chu R, Li C, Wang S, et al. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol 2014;9:1175-82. [Crossref] [PubMed]
- Anema SG, Lee SK, Lowe EK, et al. Rheological properties of acid gels prepared from heated pH-adjusted skim milk. J Agric Food Chem 2004;52:337-43. [Crossref] [PubMed]
- Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol 2010;5:1165-73. [Crossref] [PubMed]
- Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 2010;77:536-42. [Crossref] [PubMed]
- Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015;87:62-73. [Crossref] [PubMed]
- Makris K, Spanou L. Acute Kidney Injury: Diagnostic Approaches and Controversies. Clin Biochem Rev 2016;37:153-75. [PubMed]
- Cruz DN, Ricci Z, Ronco C. Clinical review: RIFLE and AKIN--time for reappraisal. Crit Care 2009;13:211. [Crossref] [PubMed]
- Gaiao S, Cruz DN. Baseline creatinine to define acute kidney injury: is there any consensus? Nephrol Dial Transplant 2010;25:3812-4. [Crossref] [PubMed]
- Abazov VM, Abbott B, Abolins M, et al. Search for doubly charged higgs boson pair production in the decay to mu(+)mu(+)mu(-)mu(-) in pp collisions at sqrt[s]=1.96 TeV. Phys Rev Lett 2004;93:141801 [Crossref] [PubMed]
- Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am 2005;89:457-73. [Crossref] [PubMed]
- Stevens LA, Coresh J, Greene T, et al. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473-83. [Crossref] [PubMed]
- Delanaye P. How Measuring Glomerular Filtration Rate? Comparison of Reference Methods. In: Sahay M. editor. Basic Nephrology and Acute Kidney Injury. IntechOpen, 2012.
- Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014;63:820-34. [Crossref] [PubMed]
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 1992;38:1933-53. [PubMed]
- Bauer JH, Brooks CS, Burch RN. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am J Kidney Dis 1982;2:337-46. [Crossref] [PubMed]
- Shemesh O, Golbetz H, Kriss JP, et al. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 1985;28:830-8. [Crossref] [PubMed]
- Payne RB. Creatinine clearance: a redundant clinical investigation. Ann Clin Biochem 1986;23:243-50. [Crossref] [PubMed]
- van Acker BA, Koomen GC, Koopman MG, et al. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 1992;340:1326-9. [Crossref] [PubMed]
- Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 2008;3:348-54. [Crossref] [PubMed]
- Patel SS, Molnar MZ, Tayek JA, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle 2013;4:19-29. [Crossref] [PubMed]
- Delanaye P, Cavalier E, Pottel H. Serum Creatinine: Not So Simple! Nephron 2017;136:302-8. [Crossref] [PubMed]
- Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol 2009;20:1217-21. [Crossref] [PubMed]
- Schetz M, Gunst J, Van den Berghe G. The impact of using estimated GFR versus creatinine clearance on the evaluation of recovery from acute kidney injury in the ICU. Intensive Care Med 2014;40:1709-17. [Crossref] [PubMed]
- Chiou WL, Hsu FH. Pharmacokinetics of creatinine in man and its implications in the monitoring of renal function and in dosage regimen modifications in patients with renal insufficiency. J Clin Pharmacol 1975;15:427-34. [Crossref] [PubMed]
- Delanaye P, Mariat C, Cavalier E, et al. Trimethoprim, creatinine and creatinine-based equations. Nephron Clin Pract 2011;119:c187-93; discussion c193-4.
- Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006;52:5-18. [Crossref] [PubMed]
- Peake M, Whiting M. Measurement of serum creatinine--current status and future goals. Clin Biochem Rev 2006;27:173-84. [PubMed]
- Pieroni L, Delanaye P, Boutten A, et al. A multicentric evaluation of IDMS-traceable creatinine enzymatic assays. Clin Chim Acta 2011;412:2070-5. [Crossref] [PubMed]
- Boutten A, Bargnoux AS, Carlier MC, et al. Enzymatic but not compensated Jaffe methods reach the desirable specifications of NKDEP at normal levels of creatinine. Results of the French multicentric evaluation. Clin Chim Acta 2013;419:132-5. [Crossref] [PubMed]
- Hoste L, Deiteren K, Pottel H, et al. Routine serum creatinine measurements: how well do we perform? BMC Nephrol 2015;16:21. [Crossref] [PubMed]
- Cobbaert CM, Baadenhuijsen H, Weykamp CW. Prime time for enzymatic creatinine methods in pediatrics. Clin Chem 2009;55:549-58. [Crossref] [PubMed]
- Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int 1985;27:928-37. [Crossref] [PubMed]
- Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care 2010;14:R82. [Crossref] [PubMed]
- Liu KD, Thompson BT, Ancukiewicz M, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 2011;39:2665-71. [Crossref] [PubMed]
- Pickering JW, Ralib AM, Endre ZH. Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care 2013;17:R7. [Crossref] [PubMed]
- Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013;61:649-72. [Crossref] [PubMed]
- Ostermann M. Diagnosis of acute kidney injury: Kidney Disease Improving Global Outcomes criteria and beyond. Curr Opin Crit Care 2014;20:581-7. [Crossref] [PubMed]
- Molitoris BA, Reilly ES. Quantifying Glomerular Filtration Rates in Acute Kidney Injury: A Requirement for Translational Success. Semin Nephrol 2016;36:31-41. [Crossref] [PubMed]
- Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med 2008;36:1129-37. [Crossref] [PubMed]
- Newsome BB, Warnock DG, McClellan WM, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 2008;168:609-16. [Crossref] [PubMed]
- Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 2011;171:226-33. [Crossref] [PubMed]
- Cruz DN, Ferrer-Nadal A, Piccinni P, et al. Utilization of small changes in serum creatinine with clinical risk factors to assess the risk of AKI in critically lll adults. Clin J Am Soc Nephrol 2014;9:663-72. [Crossref] [PubMed]
- Liotta M, Olsson D, Sartipy U, et al. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am J Cardiol 2014;113:70-5. [Crossref] [PubMed]
- Fraser C. Biological Variation: From Principles to Practice. Washington, DC: AACC Press, 2001.
- Oosterhuis WP. Analytical performance specifications in clinical chemistry: the holy grail? J Lab Precis Med 2017;2:7. [Crossref]
- Sottas PE, Kapke GF, Leroux JM. Adaptive Bayesian analysis of serum creatinine as a marker for drug-induced renal impairment in an early-phase clinical trial. Clin Chem 2012;58:1592-6. [Crossref] [PubMed]
- Sottas PE, Kapke GF, Leroux JM. Adaptive Bayesian approach to clinical trial renal impairment biomarker signal from urea and creatinine. Int J Biol Sci 2013;9:156-63. [Crossref] [PubMed]
- Fraser CG, Williams P. Short-term biological variation of plasma analytes in renal disease. Clin Chem 1983;29:508-10. [PubMed]
- Holzel WG. Intra-individual variation of analytes in serum from patients with chronic liver diseases. Clin Chem 1987;33:1133-6. [PubMed]
- Holzel WG. Intra-individual variation of some analytes in serum of patients with chronic renal failure. Clin Chem 1987;33:670-3. [PubMed]
- Holzel WG. Intra-individual variation of some analytes in serum of patients with insulin-dependent diabetes mellitus. Clin Chem 1987;33:57-61. [PubMed]
- Ricos C, Iglesias N, Garcia-Lario JV, et al. Within-subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem 2007;44:343-52. [Crossref] [PubMed]
- Carter JL, Parker CT, Stevens PE, et al. Biological Variation of Plasma and Urinary Markers of Acute Kidney Injury in Patients with Chronic Kidney Disease. Clin Chem 2016;62:876-83. [Crossref] [PubMed]
- Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409-37. [Crossref] [PubMed]
- Fokkema MR, Herrmann Z, Muskiet FA, et al. Reference change values for brain natriuretic peptides revisited. Clin Chem 2006;52:1602-3. [Crossref] [PubMed]
- Ceriotti F, Boyd JC, Klein G, et al. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem 2008;54:559-66. [Crossref] [PubMed]
- Braga F, Ferraro S, Ieva F, et al. A new robust statistical model for interpretation of differences in serial test results from an individual. Clin Chem Lab Med 2015;53:815-22. [Crossref] [PubMed]
- Lund F, Petersen PH, Fraser CG, et al. Calculation of limits for significant bidirectional changes in two or more serial results of a biomarker based on a computer simulation model. Ann Clin Biochem 2015;52:434-40. [Crossref] [PubMed]
- Lund F, Petersen PH, Fraser CG, et al. Calculation of limits for significant unidirectional changes in two or more serial results of a biomarker based on a computer simulation model. Ann Clin Biochem 2015;52:237-44. [Crossref] [PubMed]
- Makris K, Stefani D, Makri E, et al. Evaluation of a particle enhanced turbidimetric assay for the measurement of neutrophil gelatinase-associated lipocalin in plasma and urine on Architect-8000: Analytical performance and establishment of reference values. Clin Biochem 2015;48:1291-7. [Crossref] [PubMed]
- Carobene A, Braga F, Roraas T, et al. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and gamma-glutamyl transferase. Clin Chem Lab Med 2013;51:1997-2007. [Crossref] [PubMed]
- Bartlett WA, Braga F, Carobene A, et al. A checklist for critical appraisal of studies of biological variation. Clin Chem Lab Med 2015;53:879-85. [Crossref] [PubMed]
- Simundic AM, Kackov S, Miler M, et al. Terms and symbols used in studies on biological variation: the need for harmonization. Clin Chem 2015;61:438-9. [Crossref] [PubMed]
- Ricos C, Alvarez V, Cava F, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491-500. [Crossref] [PubMed]
- Perich C, Minchinela J, Ricos C, et al. Biological variation database: structure and criteria used for generation and update. Clin Chem Lab Med 2015;53:299-305. [Crossref] [PubMed]
- Ricos C, Alvarez V, Perich C, et al. Rationale for using data on biological variation. Clin Chem Lab Med 2015;53:863-70. [Crossref] [PubMed]
- Lehner GF, Forni LG, Joannidis M. Oliguria and Biomarkers of Acute Kidney Injury: Star Struck Lovers or Strangers in the Night? Nephron 2016;134:183-90. [Crossref] [PubMed]
- Prowle JR, Liu YL, Licari E, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care 2011;15:R172. [Crossref] [PubMed]
- Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by Urine Output versus Serum Creatinine Level. J Am Soc Nephrol 2015;26:2231-8. [Crossref] [PubMed]
- Cerda J. Oliguria: an earlier and accurate biomarker of acute kidney injury? Kidney Int 2011;80:699-701. [Crossref] [PubMed]
- Legrand M, Payen D. Understanding urine output in critically ill patients. Ann Intensive Care 2011;1:13. [Crossref] [PubMed]
- Macedo E, Malhotra R, Bouchard J, et al. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int 2011;80:760-7. [Crossref] [PubMed]
- Selby NM, Fluck RJ, Kolhe NV, et al. International Criteria for Acute Kidney Injury: Advantages and Remaining Challenges. PLoS Med 2016;13:e1002122 [Crossref] [PubMed]
- Macedo E, Malhotra R, Claure-Del Granado R, et al. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2011;26:509-15. [Crossref] [PubMed]
- Wlodzimirow KA, Abu-Hanna A, Slabbekoorn M, et al. A comparison of RIFLE with and without urine output criteria for acute kidney injury in critically ill patients. Crit Care 2012;16:R200. [Crossref] [PubMed]
- Md Ralib A, Pickering JW, Shaw GM, et al. The urine output definition of acute kidney injury is too liberal. Crit Care 2013;17:R112. [Crossref] [PubMed]
- Vanmassenhove J, Glorieux G, Hoste E, et al. Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care 2013;17:R234. [Crossref] [PubMed]
- Leedahl DD, Frazee EN, Schramm GE, et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clin J Am Soc Nephrol 2014;9:1168-74. [Crossref] [PubMed]
- Jelliffe R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol 2002;22:320-4. [Crossref] [PubMed]
- Bouchard J, Macedo E, Soroko S, et al. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol Dial Transplant 2010;25:102-7. [Crossref] [PubMed]
- Rabito CA, Panico F, Rubin R, et al. Noninvasive, real-time monitoring of renal function during critical care. J Am Soc Nephrol 1994;4:1421-8. [PubMed]
- Pickering JW, Frampton CM, Walker RJ, et al. Four hour creatinine clearance is better than plasma creatinine for monitoring renal function in critically ill patients. Crit Care 2012;16:R107. [Crossref] [PubMed]
- Soveri I, Berg UB, Bjork J, et al. Measuring GFR: a systematic review. Am J Kidney Dis 2014;64:411-24. [Crossref] [PubMed]
- Delanaye P, Ebert N, Melsom T, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J 2016;9:682-99. [Crossref] [PubMed]
- Delanaye P, Melsom T, Ebert N, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J 2016;9:700-4. [Crossref] [PubMed]
- Dixon JJ, Lane K, Dalton RN, et al. Validation of a continuous infusion of low dose Iohexol to measure glomerular filtration rate: randomised clinical trial. J Transl Med 2015;13:58. [Crossref] [PubMed]
- Dixon JJ, Lane K, Dalton RN, et al. Continuous Infusion of Low-Dose Iohexol Measures Changing Glomerular Filtration Rate in Critically Ill Patients. Crit Care Med 2018;46:e190-7. [Crossref] [PubMed]
- Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012-24. [Crossref] [PubMed]
- Pepin MN, Bouchard J, Legault L, et al. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007;50:566-73. [Crossref] [PubMed]
- Diskin CJ, Stokes TJ, Dansby LM, et al. Toward the optimal clinical use of the fraction excretion of solutes in oliguric azotemia. Ren Fail 2010;32:1245-54. [Crossref] [PubMed]
- Prowle J, Bagshaw SM, Bellomo R. Renal blood flow, fractional excretion of sodium and acute kidney injury: time for a new paradigm? Curr Opin Crit Care 2012;18:585-92. [Crossref] [PubMed]
- Fenske W, Stork S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab 2008;93:2991-7. [Crossref] [PubMed]
- Hall IE, Coca SG, Perazella MA, et al. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol 2011;6:2740-9. [Crossref] [PubMed]
- Langenberg C, Wan L, Bagshaw SM, et al. Urinary biochemistry in experimental septic acute renal failure. Nephrol Dial Transplant 2006;21:3389-97. [Crossref] [PubMed]
- Langenberg C, Wan L, Egi M, et al. Renal blood flow in experimental septic acute renal failure. Kidney Int 2006;69:1996-2002. [Crossref] [PubMed]
- Bagshaw SM, Langenberg C, Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis 2006;48:695-705. [Crossref] [PubMed]
- Bagshaw SM, Langenberg C, Wan L, et al. A systematic review of urinary findings in experimental septic acute renal failure. Crit Care Med 2007;35:1592-8. [Crossref] [PubMed]
- Bagshaw SM, Gibney RT. Acute kidney injury: clinical value of urine microscopy in acute kidney injury. Nat Rev Nephrol 2009;5:185-6. [Crossref] [PubMed]
- Maciel AT, Vitorio D. Urine biochemistry assessment in critically ill patients: controversies and future perspectives. J Clin Monit Comput 2017;31:539-46. [Crossref] [PubMed]
- Maciel AT, Park M. Urine assessment in the critically ill: a matter of both quantity and quality. Rev Bras Ter Intensiva 2013;25:184-5. [Crossref] [PubMed]
- Maciel AT, Park M, Macedo E. Physicochemical analysis of blood and urine in the course of acute kidney injury in critically ill patients: a prospective, observational study. BMC Anesthesiol 2013;13:31. [Crossref] [PubMed]
- Maciel AT, Vitorio D. Urine biochemistry in the early postoperative period after cardiac surgery: role in acute kidney injury monitoring. Case Rep Crit Care 2013;2013:103450 [Crossref] [PubMed]
- Bentley B. A review of methods to measure dietary sodium intake. J Cardiovasc Nurs 2006;21:63-7. [Crossref] [PubMed]
- McLean RM. Measuring population sodium intake: a review of methods. Nutrients 2014;6:4651-62. [Crossref] [PubMed]
- Mente A, O'Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J Hypertens 2014;32:1005-14; discussion 1015. [Crossref] [PubMed]
- Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press 2010;8:72-81. [Crossref] [PubMed]
- Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care 2013;28:216 e11-20.
- Hoorn EJ, Betjes MG, Weigel J, et al. Hypernatraemia in critically ill patients: too little water and too much salt. Nephrol Dial Transplant 2008;23:1562-8. [Crossref] [PubMed]
- Besen BA, Gobatto AL, Melro LM, et al. Fluid and electrolyte overload in critically ill patients: An overview. World J Crit Care Med 2015;4:116-29. [Crossref] [PubMed]
- Kahn T. Hypernatremia with edema. Arch Intern Med 1999;159:93-8. [Crossref] [PubMed]
- Sam R, Hart P, Haghighat R, et al. Hypervolemic hypernatremia in patients recovering from acute kidney injury in the intensive care unit. Clin Exp Nephrol 2012;16:136-46. [Crossref] [PubMed]
- Sarahian S, Pouria MM, Ing TS, et al. Hypervolemic hypernatremia is the most common type of hypernatremia in the intensive care unit. Int Urol Nephrol 2015;47:1817-21. [Crossref] [PubMed]
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976;236:579-81. [Crossref] [PubMed]
- Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 1978;89:47-50. [Crossref] [PubMed]
- Sodium homeostasis in chronic renal disease. Kidney Int 1982;21:886-97. [Crossref] [PubMed]
- Gotfried J, Wiesen J, Raina R, et al. Finding the cause of acute kidney injury: which index of fractional excretion is better? Cleve Clin J Med 2012;79:121-6. [Crossref] [PubMed]
- Fang LS, Sirota RA, Ebert TH, et al. Low fractional excretion of sodium with contrast media-induced acute renal failure. Arch Intern Med 1980;140:531-3. [Crossref] [PubMed]
- Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med 1983;143:738-9. [Crossref] [PubMed]
- Corwin HL, Schreiber MJ, Fang LS. Low fractional excretion of sodium. Occurrence with hemoglobinuric- and myoglobinuric-induced acute renal failure. Arch Intern Med 1984;144:981-2. [Crossref] [PubMed]
- Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med 1985;145:108-12. [Crossref] [PubMed]
- Diskin CJ, Stokes TJ, Dansby LM, et al. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 2010;114:c145-50. [Crossref] [PubMed]
- Bagshaw SM, Bennett M, Devarajan P, et al. Urine biochemistry in septic and non-septic acute kidney injury: a prospective observational study. J Crit Care 2013;28:371-8. [Crossref] [PubMed]
- Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol 2012;7:167-74. [Crossref] [PubMed]
- Materson BJ. Insights into intrarenal sites and mechanisms of action of diuretic agents. Am Heart J 1983;106:188-208. [Crossref] [PubMed]
- Hropot M, Fowler N, Karlmark B, et al. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int 1985;28:477-89. [Crossref] [PubMed]
- Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992;12:49-54. [Crossref] [PubMed]
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002;62:2223-9. [Crossref] [PubMed]
- Darmon M, Vincent F, Dellamonica J, et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care 2011;15:R178. [Crossref] [PubMed]
- Pons B, Lautrette A, Oziel J, et al. Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: multicenter cohort study. Crit Care 2013;17:R56. [Crossref] [PubMed]
- Musch W, Verfaillie L, Decaux G. Age-related increase in plasma urea level and decrease in fractional urea excretion: clinical application in the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol 2006;1:909-14. [Crossref] [PubMed]
- Espinel CH, Gregory AW. Differential diagnosis of acute renal failure. Clin Nephrol 1980;13:73-7. [PubMed]
- Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med 2002;137:744-52. [Crossref] [PubMed]
- Claure-Del Granado R, Macedo E, Mehta RL. Urine microscopy in acute kidney injury: time for a change. Am J Kidney Dis 2011;57:657-60. [Crossref] [PubMed]
- Cameron JS. A history of urine microscopy. Clin Chem Lab Med 2015;53:s1453-64. [Crossref] [PubMed]
- Bunjevac A, Gabaj NN, Miler M, et al. Preanalytics of urine sediment examination: effect of relative centrifugal force, tube type, volume of sample and supernatant removal. Biochem Med (Zagreb) 2018;28:010707 [Crossref] [PubMed]
- Becker GJ, Garigali G, Fogazzi GB. Advances in Urine Microscopy. Am J Kidney Dis 2016;67:954-64. [Crossref] [PubMed]
- Aspevall O, Hallander H, Gant V, et al. European guidelines for urinalysis: a collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin Microbiol Infect 2001;7:173-8. [Crossref] [PubMed]
- Perazella MA. The urine sediment as a biomarker of kidney disease. Am J Kidney Dis 2015;66:748-55. [Crossref] [PubMed]
- Perazella MA, Parikh CR. How can urine microscopy influence the differential diagnosis of AKI? Clin J Am Soc Nephrol 2009;4:691-3. [Crossref] [PubMed]
- Chawla LS, Dommu A, Berger A, et al. Urinary sediment cast scoring index for acute kidney injury: a pilot study. Nephron Clin Pract 2008;110:c145-50. [Crossref] [PubMed]
- Perazella MA, Coca SG, Kanbay M, et al. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008;3:1615-9. [Crossref] [PubMed]
- Bagshaw SM, Haase M, Haase-Fielitz A, et al. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol Dial Transplant 2012;27:582-8. [Crossref] [PubMed]
- Kanbay M, Kasapoglu B, Perazella MA. Acute tubular necrosis and pre-renal acute kidney injury: utility of urine microscopy in their evaluation- a systematic review. Int Urol Nephrol 2010;42:425-33. [Crossref] [PubMed]
- Perazella MA, Coca SG, Hall IE, et al. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2010;5:402-8. [Crossref] [PubMed]
- Tsai JJ, Yeun JY, Kumar VA, et al. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005;46:820-9. [Crossref] [PubMed]
- Fogazzi GB, Garigali G, Pirovano B, et al. How to improve the teaching of urine microscopy. Clin Chem Lab Med 2007;45:407-12. [Crossref] [PubMed]
- Chien TI, Kao JT, Liu HL, et al. Urine sediment examination: a comparison of automated urinalysis systems and manual microscopy. Clin Chim Acta 2007;384:28-34. [Crossref] [PubMed]
- Chien TI, Lu JY, Kao JT, et al. Comparison of three automated urinalysis systems--Bayer Clinitek Atlas, Roche Urisys 2400 and Arkray Aution Max for testing urine chemistry and detection of bacteriuria. Clin Chim Acta 2007;377:98-102. [Crossref] [PubMed]
- Sharda N, Bakhtar O, Thajudeen B, et al. Manual urine microscopy versus automated urine analyzer microscopy in patients with acute kidney injury. Lab Med 2014;45:e152-5. [Crossref] [PubMed]
- Selby NM. Electronic alerts for acute kidney injury. Curr Opin Nephrol Hypertens 2013;22:637-42. [Crossref] [PubMed]
- Horne KL, Selby NM. Recent developments in electronic alerts for acute kidney injury. Curr Opin Crit Care 2015;21:479-84. [PubMed]
- Haase M, Kribben A, Zidek W, et al. Electronic Alerts for Acute Kidney Injury. Dtsch Arztebl Int 2017;114:1-8. [PubMed]
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist management of acute renal failure. QJM 2001;94:533-40. [Crossref] [PubMed]
- Mehta RL, McDonald B, Gabbai F, et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med 2002;113:456-61. [Crossref] [PubMed]
- James MT, Wald R, Bell CM, et al. Weekend hospital admission, acute kidney injury, and mortality. J Am Soc Nephrol 2010;21:845-51. [Crossref] [PubMed]
- Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diawgnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 2012;7:533-40. [Crossref] [PubMed]
Cite this article as: Makris K. The role of the clinical laboratory in the detection and monitoring of acute kidney injury. J Lab Precis Med 2018;3:69.


