
Head-to-head comparison of three different high-sensitivity cardiac troponin assays for early rule-in and rule-out of acute myocardial infarction
The blood-based biomarkers cardiac troponin T and cardiac troponin I are used by clinicians to aid the diagnosis of acute myocardial infarction (AMI) and any other myocardial injury (1,2). The first assays for measurement of cardiac troponin plasma concentrations were developed in the early 1990s. Since then, continuous efforts were undertaken to improve the analytical precision of cardiac troponin assays at low analyte concentrations (2). According to current guidelines, the analytical coefficient of variation (CV) of cardiac troponin assays should be <10% at the 99th percentile upper reference limit (URL) of a normal reference population (2). In addition, the same guidelines claim that cardiac troponin assays should measure analyte concentrations above the limit of detection (LOD) in ≥50% of a normal reference population (2). If cardiac troponin assays are able to meet these two requirements, they are termed high-sensitivity cardiac troponin T (hs-cTnT) and high-sensitivity cardiac troponin I (hs-cTnI) assays (2).
In clinical routines, hs-cTnT and hs-cTnI assays are used for both: (I) for the diagnosis of myocardial injury and (II) for the differentiation of chronic cardiac disease from acute cardiac presentations (3). Of note, as detailed in Table 1, AMI is only one reason for myocardial injury and, thus, for increased plasma concentrations of hs-cTnT and hs-cTnI (1,3). For the diagnosis of AMI, clinicians have to consider the patients’ clinical presentation, the electrocardiogram and imaging studies, as well as the plasma concentrations of cardiac troponin (1). Especially in the emergency department (ED), clinicians try to exclude AMI rapidly (3), and compared to using the older cardiac troponin assays, using hs-cTnT and hs-cTnI assays decreases the time for ruling out AMI (3). Besides an increased cardiac troponin concentration above the 99th percentile URL, changing cardiac troponin concentrations over time by serial measurements are a “condicio sine qua non” for diagnosing AMI (1-3).
Table 1
| Acute myocardial infarction |
| Tachyarrhythmias |
| Heart failure |
| Hypertensive emergencies |
| Critical illness (e.g., shock/sepsis/burns) |
| Myocarditis |
| Tako-Tsubo cardiomyopathy |
| Structural heart disease (e.g., aortic stenosis) |
| Aortic dissection |
| Pulmonary embolism, pulmonary hypertension |
| Renal dysfunction and associated cardiac disease |
| Coronary spasm |
| Acute neurological event (e.g., stroke or subarachnoid haemorrhage) |
| Cardiac contusion or cardiac procedures (e.g., coronary artery bypass surgery, percutaneous coronary intervention, ablation, pacing, cardioversion, or endomyocardial biopsy) |
| Hypo- and hyperthyroidism |
| Infiltrative diseases (e.g., amyloidosis, haemochromatosis, sarcoidosis, scleroderma) |
| Myocardial drug toxicity or poisoning (e.g., doxorubicin, 5-flourouracil, herceptin, snake venoms) |
| Extreme endurance efforts |
| Rhabdomyolysis |
Modified with permission from reference (4).
Currently, there are one hs-cTnT assay (Roche) and six hs-cTnI assays (Abbott, Beckman Coulter, BioMerieux, Pathfast, Siemens and Singulex) commercially available. Of them, two assays, namely the Roche hs-cTnT assay (Elecsys platform) and the Abbott hs-cTnI assay (Architect platform), have been extensively investigated in large diagnostic studies including the successful derivation and validation of early 0/1-hour and 0/2-hour triage algorithms (5). The results of evaluation studies of the other assays were published less frequently, and, thus, further diagnostic studies including head-to head comparisons of the currently available hs-cTnT and hs-cTnI assays would be welcome. Consequently, a recent study by Boeddinghaus et al. (5) validating the Siemens hs-cTnI assay for early diagnosis of AMI and comparing its performance with the Roche and the Abbott assays is an important step forwards to increase our knowledge on the commercially available hs-cTnI assays.
As described in the package insert, the Siemens hs-cTnI assay has a sex-neutral 99th percentile URL of 59 ng/L for plasma samples with a corresponding analytical CV of <5%. The sex-specific 99th percentile URLs are 79 ng/L for plasma samples of male individuals and 54 ng/L for plasma samples of female individuals. The measurement range of the assay is 3–25,000 ng/L, the LOD was found to be 2 ng/L, and the limit of quantification is 3 ng/L. The 10% total analytical CV was obtained at 10 ng/L. Accordingly, this assay meets the current recommendations for hs-cTnI assays (2). The Siemens hs-cTnI assay is a homogeneous, sandwich chemiluminescent immunoassay. The time to the first result is 18 minutes.
In their study (5), Boeddinghaus et al. enrolled patients ≥18 years of age presenting to the ED with symptoms suggestive of AMI such as acute chest discomfort and angina pectoris with a symptom onset or peak within the last 12 hours. Boeddinghaus et al. excluded patients with terminal kidney failure on chronic dialysis, patients with ST-segment elevation myocardial infarction (STEMI) and patients in whom the diagnosis remained obscure. In the 1,755 study participants, the final diagnoses were adjudicated by two independent cardiologists including all clinical information as well as (I) the results of serial hs-cTnT measurements with the Roche assay (for the primary analysis of this study); and (II) the results of serial hs-cTnI measurements with the Abbott assay (for the secondary analysis of this study). Thus, the “reference standard” for the final diagnosis of AMI was the clinical information plus serial Roche hs-cTnT for the primary analysis, and the clinical information plus serial Abbott hs-cTnI for the secondary analysis. Siemens hs-cTnI was the “index test” for the early diagnosis of AMI and was measured from frozen blood samples obtained at the patients’ presentation in the ED and 1 hour and 2 hours afterwards. AMI was the final diagnosis in 318 of 1,755 patients in the primary analysis (using Roche hs-cTnT), and in 299 of 1,755 patients in the secondary analysis (using Abbott hs-cTnI).
Using the previously described primary and secondary analyses, Boeddinghaus et al. compared the diagnostic utility of Siemens hs-cTnI, Roche hs-cTnT and Abbott hs-cTnI obtained at the patients’ presentation in the ED (baseline values) for the final diagnosis of AMI. As a result, the baseline values of all three assays had comparable diagnostic accuracies (as quantified by area under the ROC curves)—the area under the ROC curve for all three assays was between 0.93 and 0.95, with overlapping 95% confidence intervals. Furthermore, Boeddinghaus et al. investigated, whether the concept of an early 1-hour diagnosis to either rule out or rule in AMI is working with specific Siemens hs-cTnI algorithms. For this purpose, the authors subdivided their cohort in a derivation cohort and a validation cohort. With this approach, they found that applying the derived Siemens hs-cTnI 0/1-hour algorithm (using the baseline value and the analyte kinetics within the first hour) to the validation cohort, 46% of the patients were ruled out (sensitivity, approx. 99%) and 18% of the patients were ruled in (specificity, approx. 94%) in the previously described primary and secondary analyses. Again, the results obtained with the Siemens hs-cTnI assay were roughly comparable to those obtained with the Roche hs-cTnT and Abbott hs-cTnI assays. Based on these results, Boeddinghaus et al. concluded, that the diagnostic accuracy and the clinical utility of the Siemens hs-cTnI assay is adequate and comparable to the Roche hs-cTnT and Abbott hs-cTnI assays (5).
Although the findings by Boeddinghaus et al. have substantial implications for the use of the Siemens hs-cTnI assay in clinical routines, some issues deserve closer attention. This is a prospective, international multicentre study with 12 centers in five countries, and the patients were recruited from 2006 to 2013 (i.e., enrolment of not more than 250–300 patients per year). The work by Boeddinghaus et al. refers to patients with acute chest discomfort under the conditions of a prospectively conducted study (including the procedure of informed signed consent of the patients). However, we believe that this might not accurately reflect the practice of cardiac troponin testing in “real life use”. For example, the median age of the chest pain patients in the study by Boeddinghaus et al. was 62 years, whereas it is usually >65 years in patients presenting with suspected non-ST-elevation myocardial infarction (NSTEMI) in clinical routines. Especially the elderly might present to the ED with atypical symptoms, with critical illness, with a higher proportion of diabetes mellitus or with end stage renal disease. Non-consideration of such patients could have changed the proportion of ruling in and ruling out AMI in the study by Boeddinghaus et al. compared to the situation in clinical practice.
Furthermore, we would like to emphasize, that the study of Boeddinghaus et al. investigated the utility of the Siemens hs-cTnI assay for the early rule in or rule out of AMI in ED patients. In this context, these algorithms should be applied only after STEMI has been ruled out by the electrocardiogram performed a presentation (as done by the authors). Anyhow, the study of Boeddinghaus et al. was not designed to evaluate the value of Siemens hs-cTnI for the final diagnosis of AMI. The protocol of Boeddinghaus et al. rather identified or excluded (acute) myocardial injury at a very early stage (i.e., at the patients’ presentation in the ED or 1–2 hours afterwards). Hence, further studies are needed demonstrating that the Siemens hs-cTnI assay is also useful for the diagnosis of AMI in conjunction with the patients’ clinical presentation, electrocardiogram and imaging studies. Although we assume that this will be possible with the Siemens hs-cTnI assay, the evaluation of concordant and discordant final diagnoses of AMI compared to the Roche hs-cTnT and Abbott hs-cTnI assays would be welcome and very useful for adopting the Siemens assay into clinical practice.
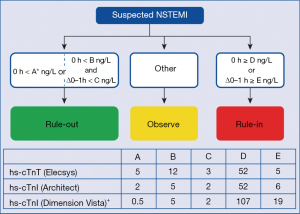
In conclusion, the current study by Boeddinghaus et al. (5) supports the concept that—particularly in low-risk patients presenting to an ED—AMI can be excluded at an early stage (i.e., during the first 1–2 hours after the patients’ presentation in an ED) with high sensitivity cardiac troponin assays. Respective protocols have also been endorsed by the guidelines of the European Society of Cardiology (4), as shown in Figure 1. In their work, the authors demonstrated that the value of the Siemens hs-cTnI assay for the early diagnosis of AMI in an ED setting is comparable to the respective information provided by the well-established Roche hs-cTnT and Abbott hs-cTnI assays. This information is very important for physicians using this assay in clinical practice, because the published scientific evidence on the Siemens assay was limited up to date. Nevertheless, caution is recommended when evaluating certain patient subsets in “real life use” such as patients with atypical symptoms, elderly patients, critical ill patients, and patients with advanced renal disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Xu-Hua Mao (Department of Clinical Laboratory, Yixing People’s Hospital, Wuxi, China).
Conflicts of Interest: B Dieplinger and T Mueller have received speaking fees from Abbott Diagnostics and Roche Diagnostics. S Giuliani has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231-64. [Crossref] [PubMed]
- Wu AHB, Christenson RH, Greene DN, et al. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645-55. [Crossref] [PubMed]
- Andruchow JE, Kavsak PA, McRae AD. Contemporary Emergency Department Management of Patients with Chest Pain: A Concise Review and Guide for the High-Sensitivity Troponin Era. Can J Cardiol 2018;34:98-108. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Boeddinghaus J, Twerenbold R, Nestelberger T, et al. Clinical Validation of a Novel High-Sensitivity Cardiac Troponin I Assay for Early Diagnosis of Acute Myocardial Infarction. Clin Chem 2018;64:1347-60. [Crossref] [PubMed]
Cite this article as: Giuliani S, Dieplinger B, Mueller T. Head-to-head comparison of three different high-sensitivity cardiac troponin assays for early rule-in and rule-out of acute myocardial infarction. J Lab Precis Med 2019;4:4.


