
Comparison of cardiac biomarker dynamics in marathon, semi-marathon and untrained runners: what is the impact on results interpretation?
Introduction
Regular physical exercise is recommended for the primary prevention of cardiovascular (CV) disease (1). In recent years, participation in competitions such as marathon, semi-marathon and cycling events has become increasingly popular. There is a small number of high-performance professional athletes between 18 and 35 years physically well monitored and medically supervised, however the number of non-professional athletes with individuals of all age groups is increasing. Physical exercise has a clear overall benefit, but there is a small but significant increase in the risk of sudden cardiac death during or shortly after vigorous exercise (2) especially in older athletes (3).
Data on the long-term effect of vigorous exercise on CV risk in presumably healthy individuals participating in marathons or half marathons are limited. It is important to look at the benefit of exercise training, but also try to evaluate possible negative effect of extreme physical exercise.
There are several studies on cardiac biomarker changes. Mild to moderate elevations in those markers have been described as a result of a running exercise (4,5). Exact underlying mechanism for these biomarker elevations reflecting physiological or even pathophysiological changes is unknown and less trained athletes might exhibit a higher cardiac risk compared to well-trained runners.
High-sensitivity cardiac troponin (cTn) assays are now available that can reliably measure cTn levels in low concentrations seen in healthy individuals (6). Detectable cTn levels determined with high sensitivity troponin assays in athletes have been reported from different studies (7,8). Additional marker for potential cardiac stretch and potential myocardial fibrotic processes during endurance training are natriuretic peptides (NPs) and Galectin-3 (Gal-3) (9).
The aim of this study was to evaluate cardiac biomarker levels in well trained athletes, healthy runners, completing a marathon or semi marathon compared to a control group consisting of healthy, untrained individuals completing 1 hour of running and to highlight the impact on the lab results interpretation in emergency department.
Methods
Subjects
Included in the study were healthy male marathon (n=23), semi-marathon runners (n=15) and a control group of untrained runners (n=17). The exclusion criterion was “no history of cardiovascular disease”. The marathon runners were well trained athletes with a weekly training plan of 5 h 28 min ±2 h 33 min, semi-marathon runners weekly training plan was 4 h 22 min ±1 h 29 min. Weekly exercise in the control group of untrained runners was below 2 h. Training levels and training duration of the control group corresponds to the general recommendation for weekly exercise.
Running exercise duration was different for the different runner groups. Median duration to complete the marathon run was 3 h 50 min 48 sec (±27 min 30 sec) and 1 h 55 min 18 sec (±15 min 31 sec) for the semi-marathon. Control group was asked to run for 1 hour in an athletic stadium being at their limit at the end of the exercise.
Samples
For all participants, a blood sample was taken before the beginning of the exercise (pre-exercise T0), directly (T post) and 3 hours (T 3 h post) after end of the exercise.
Blood samples (EDTA-, Lithium Heparin-plasma and serum) were centrifuged immediately after the draw for 10 minutes at 2,500 g, aliquoted and stored frozen at −80 °C before further analysis. The heart rate and the blood pressure were monitored at the same time of the blood draw.
Study was approved by Ethic committee of the University of Liege. All the subjects signed an informed consent.
Biomarkers determination
Hematocrit and hemoglobin levels were determined at all 3 time points to correct for possible post exercise dehydration.
All the biomarkers, cardiac biomarkers: high sensitivity (hs) cTnI and hs cTnT, B-type natriuretic peptide (BNP) and N-terminal Pro BNP (NT-ProBNP), creatin kinase (CK), creatin kinase-isoenzym MB (CKMB), myoglobin (MYO) and Gal-3, inflammation and renal biomarkers: C-reactive protein (CRP), myeloperoxydase (MPO), creatinine (Crea) and cystatin C (CysC), were measured in the stored aliquots.
Hs cTnI, BNP and Gal-3 were measured on the Abbott ARCHITECT i2000SR immunoanalyzers (Abbott Laboratories, Germany), hs cTnT and NT-ProBNP on the Roche Elecsys system (Roche Diagnostics, Switzerland) according to the manufacturer’s instructions for use. CK, CK MB, MYO, CRP and Crea were measured on the Roche Elecsys system (Roche Diagnostics, Switzerland). MPO was measured with a kit from Immundiagnostik (Bensheim, Germany) on an Etimax (Diasorin, Italy) and CysC was measured on a Vista (Siemens, Germany) according to the manufacturer’s instructions for use.
The analytical performances of the studied biomarkers are summarized in the Table 1 (6,10-16).
Table 1
| Parameters | LOD | LOQ or CV of 10% | 99th percentile | Recommended threshold |
|---|---|---|---|---|
| TnI | 1.9 ng/L | 5 ng/L | 26.2 ng/L | – |
| TnT | 5 ng/L | 13 ng/L | 14 ng/L | – |
| BNP | 10 ng/L | – | – | 35 ng/L |
| NT-pro | 5 ng/L | 30 ng/L | – | 125 ng/L |
| Gal-3 | – | 6 ng/L | 25.7 ng/mL (95th) | – |
| CK | 7 UI/L | 1 UI/L | 20 UI/L (95th) | 30–175 UI/L |
| CKMB | 0.1 µg/L | 1 µg/L | 6.22 µg/L | 0–6 µg/L |
| MYO | 21 µg/L | 1 µg/L | 72 µg/L (97.5th) | 28–72 µg/L |
| CRP | 0.3 µg/L | 0.6 µg/L | – | 0–6 mg/L |
| Crea | 0.06 mg/L | 0.195 mg/L | 1.18 mg/L (97.5th) | 0.73–1.18 mg/L |
| Cys C | 0.27 mg/L | 0.66 mg/L | – | 0.62–1.11 mg/L |
| MPO | 1.6 ng/mL | – | 55 ng/mL (90th) | – |
LOD, limit of detection; LOQ, limit of quantitation; CV, cardiovascular; BNP, B-type natriuretic peptide; Gal-3, galectin-3; CK, creatin kinase; CKMB, creatin kinase-isoenzym MB; MYO, myoglobin; CRP, C-reactive protein; Crea, creatinine; Cys C, cystatin C; MPO, myeloperoxydase.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0.0 and Analyse-it for Microsoft Excel (version 2.30) and JMP version 12.0. Categorical data were summarized with number and percentages. Results are generally expressed as median with 25th–75th percentiles. Comparison between the groups was performed using a Kruskal-Wallis test with Bonferroni correction. Pearson Correlation was done to determine the degree of association between 2 variables.
Results
Median age, height, weight, body mass index (BMI), heart rate and systolic blood pressure of the 3 study groups are summarized in Table 2. There were no medical problems reported before or after the exercise in any of the participating well-trained athletes and the control group. All runners completed the runs without any reported problems during or after the exercise.
Table 2
| Characteristics | Marathon (n=23) | Semi-Marathon (n=15) | Control (n=17) | P value |
|---|---|---|---|---|
| Age (years) | 41 [37–50] | 36 [30–44] | 37 [35–41] | 0.1510 |
| Gender (M/F) | M | M | M | ns |
| Height (cm) | 176 [170–183] | 179 [175–184] | 177 [172–184] | 0.3631 |
| Weight (kg) | 74 [65–80] | 74 [67–81] | 78 [67–93] | 0.6450 |
| BMI (kg/m2) | 23 [22–26] | 23 [21–24] | 24 [22–26] | 0.5069 |
| Heart rate (per min) | ||||
| T0 | 56 [49–65] | 63 [55–74] | 65 [63–81] | 0.0204# |
| T post | 98 [92–116] | 125 [102–178] | 100 [90–108] | 0.0886 |
| T 3h post | 79 [70–87] | 81[76–85] | 82 [71–91] | 0.4660 |
| P value | 0.0022# | <0.0001* | <0.0001* | |
| Arterial pressure (PAS) (mmHg) | ||||
| T0 | 120 [120–130] | 120 [110–123] | 124 [120–135] | 0.0952 |
| T post | 95 [90–103] | 110[102–113] | 116 [113–121] | <0.0001* |
| T 3 h post | 106 [98–113] | 108[105–111] | 120 [110–130] | 0.0034# |
| P value | <0.0001* | 0.0135# | 0.1435 | |
| Arterial pressure (PAD) (mmHg) | ||||
| T0 | 80 [60–80] | 70 [70–80] | 85 [75–93] | 0.0049# |
| T post | 56 [52–66] | 68[61–70] | 81 [77–88] | <0.0001* |
| T 3 h post | 69 [62–72] | 65[62–71] | 79 [72–81] | 0.0029# |
| P value | 0.2223 | 0.0229# | 0.1862 |
*, P<0.001; #, P<0.05. IQR, inter quartile range; BMI, body mass index; PAS, pulmonary arterial systolic; PAD, pulmonary arterial diastolic.
Pre-exercise levels
Cardiac biomarker levels between marathon runners and semi-marathon runners did not show a statistically significant difference in the pre-exercise samples for Troponin I and T, BNP, NT-ProBNP and Gal-3 (Table 3).
Table 3
| Athletes | T0 | T post | T 3 h post | P value |
|---|---|---|---|---|
| Median hs-TnI (ng/mL, IQR) | ||||
| Marathon (n=19) | 4.9 (2.9–7.6) | 40.2 (20.5–81.4) | 58.1 (30.1–74.3) | <0.001* |
| Semi–Marathon (n=12) | 3.5 (1.6–7.5) | 17.1 (10.2–23.8) | 56.4 (35.1–219.6) | <0.001* |
| Control (n=16) | 2.5 (1.9–3.9) | 4.0 (2.3–5.7) | 10.4 (4.8–17.0) | 0.0012# |
| P value | 0.1565 | <0.0001* | <0.0001* | |
| Median hs-TnT (ng/mL, IQR) | ||||
| Marathon (n=23) | <5 | 30.0 (17.0–43.0) | 28.0 (18.0–38.0) | <0.0001* |
| Semi-Marathon (n=) | 6.0 (5.0–7.0) | 26.0 (16.0–39.0) | 45.0 (18.0–68.0) | <0.0001* |
| Control (n=14) | <5 | 5.0 (5.0–6.0) | 8.0 (5.0–19.0) | 0.0027# |
| P value | 0.0549 | <0.0001* | <0.0001* | |
| Median BNP (ng/L, IQR) | ||||
| Marathon (n=20) | 10 [10–18] | 13 [10–21] | 13 [11–21] | 0.1838 |
| Semi-Marathon (n=15) | 10 [10–14] | 12 [10–18] | 14 [10–20] | 0.1044 |
| Control (n=16) | 11 [10–13] | 13 [10–20] | 10 [10–17] | 0.2848 |
| P value | 0.5304 | 0.9295 | 0.0813 | |
| Median NT-proBNP (ng/L, IQR) | ||||
| Marathon (n=23) | 30 [20–37) | 83 [61–109] | 70 [51–120] | <0.0001* |
| Semi-Marathon (n=15) | 18 [8–21] | 58 [47–85] | 48 [43–83] | <0.0001* |
| Control (n=17) | 29 [19–36] | 40 [32–48] | 37 [27–49] | 0.1239 |
| P value | 0.0982 | 0.0020# | 0.0038# | |
| Median Gal-3 (ng/L, IQR) | ||||
| Marathon (n=20) | 10 [9–12] | 27 [22–29] | 18 [16–21] | <0.0001* |
| Semi-Marathon (n=14) | 13 [10–16] | 23 [22–26] | 18 [15–21] | <0.0001* |
| Control (n=17) | 10 [9–13] | 14 [12–16] | 13 [11–15] | 0.0048# |
| P value | 0.0531 | <0.0001* | 0.0002* |
*, P<0.001; #, P<0.05. IQR, inter quartile range.
All untrained runners had pre-exercise cTn I and T levels below the 99th percentile except for one marathon and two semi-marathon runners with cTn levels above 99th percentile before start of the run. Elevated BNP (>35 ng/L) value was seen in one marathon and one semi-marathon runner before start of the run. NT-ProBNP levels in the pre-exercise samples of all three running groups were all below 125 ng/L, Gal-3 levels were below 95th percentile derived from healthy individuals.
Dehydration during the run
There was an average 5% increase in hemoglobin concentration between the pre-exercise sample and the sample drawn immediately after the exercise for well-trained athletes. Hemoglobin levels in the sample drawn 3 hours post-exercise were comparable to pre-run levels indicating increase in values due to dehydration effect directly after the run. This was not seen in the control group after 1-hour running. When corrected for hemoglobin concentration cardiac biomarker levels were not significantly different.
Heart rate and arterial blood pressure
Heart rate and arterial pressure of the trained athletes and the control group were measured at all 3 time points, before, directly after and 3 hours after the running exercise (Table 2). Significant lowest heart rate before the run was seen for the marathon runners, control group had the highest heart rate. Directly after the run there was a significant increase in heart rate for all groups which decreased 3 hours later, however heart rate was still higher when compared to initial value. Arterial pressure decreased in all groups after the running exercise and also stayed low 3 hours after the exercise.
Change of biomarker levels during and after the run
For all marker’s levels and the change of levels during and after the run are the highest in the marathon runner group. The lowest levels, but also the lowest effect of exercise on biomarker level increase is seen in the control group.
Median Troponin I, Troponin T, BNP, NT-ProBNP and Gal-3 levels plus interquartile range (IQR) in the 3 running groups before exercise, directly after completion of the run and 3 hours after completion are summarized in Table 3. The percentage changes of biomarker level between pre-, post and 3-hour post exercise level is summarized in Figure 1.
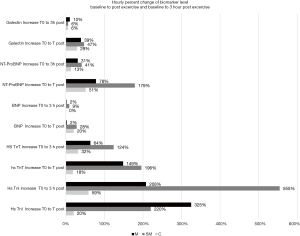
Average duration of the running exercise was 3 hours 51 minutes for the marathon, 1 hour 55 minutes for the semi-marathon and 1 hour for the untrained runners. The hourly increase of biomarker levels during the running exercise expressed as percentage of the pre-exercise baseline level is highest for cTn I (325%, 220%, 20% for marathon, semi-marathon and untrained runners), for cTn T (149%, 196% and 18%), for BNP (2%, 28% and 20%), for NT-ProBNP (78%, 179% and 51%) and for Gal-3 (39%, 47% and 29%). After completion of the running exercise NP values stabilized or decreased, however, for cTn I and T levels continued to increase.
Troponin I and T levels were significantly correlated to CK and CKMB. Troponin T values were additionally correlated to Gal-3, NT-ProBNP, BNP, CysC, Crea and MYO.
BNP and NT-Pro-BNP correlation was highly significant. NT-ProBNP however also showed significant correlation to most other markers (Gal-3, TnT, CK, CKMB CRP, CysC, Crea and MYO).
Gal-3 levels were highly correlated to TnT, NT-ProBNP, CK, CKMB, CRP, CysC, Crea and MYO (Table 4).
Table 4
| Parameters | hs TnI (ng/L) | BNP (ng/L) | Gal 3 (pg/L) | hs TnT (ng/L) | NT-ProBNP (ng/L) | MPO (µg/L) | CK (U/L) | CKMB (µg/L) | CRP (mg/L) | Cys C | Crea (mg/L) | MYO (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hs TnI (ng/L) | – | 0.0568 | 0.2674 | 0.6922# | 0.2785 | −0.1156 | 0.3037* | 0.3977* | 0.1664 | 0.0699 | 0.1551 | 0.2814 |
| BNP (ng/L) | – | – | 0.0938 | 0.1534* | 0.3613* | −0.0481 | 0.0734 | 0.0816 | 0.1679 | 0.0761 | 0.1001 | 0.1974 |
| Gal-3 (pg/L) | – | – | – | 0.5411* | 0.4349* | −0.0642 | 0.2974* | 0.3458* | 0.0109 | 0.679 | 0.7171 | 0.4297* |
| hs TnT (ng/L) | – | – | – | – | 0.5485* | −0.1146 | 0.4021* | 0.4648* | 0.2342 | 0.3567* | 0.4148* | 0.437* |
| NT-ProBNP (ng/L) | – | – | – | – | – | −0.0456 | 0.3941* | 0.3947 | 0.3319* | 0.408* | 0.3654* | 0.4557* |
| MPO (µg/L) | – | – | – | – | – | – | −0.0472* | −0.0853 | −0.0662 | −0.0656 | −0.077 | −0.0373 |
| CK (U/L) | – | – | – | – | – | – | – | 0.9324 | 0.0393 | 0.2857 | 0.2496 | 0.9267 |
| CKMB (µg/L) | – | – | – | – | – | – | – | – | 0.0809 | 0.2892 | 0.258 | 0.8837 |
| CRP (mg/L) | – | – | – | – | – | – | – | – | – | 0.0484 | 0.0435 | 0.074 |
| Cys C | – | – | – | – | – | – | – | – | – | – | 0.7935 | 0.4232* |
| Crea (mg/L) | – | – | – | – | – | – | – | – | – | – | – | 0.3995* |
*, P<0.001; #, P<0.05. BNP, B-type natriuretic peptide; Gal-3, galectin-3; CK, creatin kinase; CKMB, creatin kinase-isoenzym MB; MYO, myoglobin; CRP, C-reactive protein; Crea, creatinine; Cys C, cystatin C; MPO, myeloperoxidase.
Discussion
The aim of our study was to compare cardiac biomarker kinetics in runners at different time points depending on the exercise duration and training status of healthy athletes. Participants in the marathon and semi-marathon run were well trained with similar training levels and duration, ‘control’ group runners were selected based on a training levels of less than 2 hours per week, which was just below the general recommendation for health-enhancing physical activity, an interesting group of middle age healthy individuals’ representative of a more sedentary life style with higher risk of CV disease due to low sport activity.
Three cardiac biomarkers targeting different cardiac abnormal pathways were tested in the three running groups with different running time and training level. Cardiac T and I troponins (cTnT and cTnI) are currently regarded as reference markers of myocardial necrosis based on their excellent sensitivity and cardio specificity (17). Brain NPs are hormones synthesized by cardiomyocytes (13). High blood concentrations reflect a high myocardial afterload tension due to the stretching of the myocytes, Gal-3 is a marker of cardiac fibrosis (18,19).
Several studies looking at cardiac biomarker kinetics during different types of exercises like running, rowing or basketball playing have reported increase of biomarkers after stringent exercise (9,20)
In this study, we have seen that cTn increased in trained athletes, like marathon and semi-marathon runners during the run and continued to rise 3 hours after completion of running exercise. There is no consensus on pathophysiology and probable clinical impact of cTn elevation in athletes during and after the run. Different possible mechanisms have been proposed for Troponin release during the run including higher membrane permeability due to increased mechanical stress on the cardiomyocytes (bleb), increased production of oxidative radicals or altered acid base balance (20-24). The fact that the same Troponin release pattern during and post exercise is not only seen in trained athletes but also in the control group is of special interest. Heart rate increased and stayed high also 3 hours after completion of the run. Ischemic conditioning during and continuing after the race could cause transient, most likely reversible increased cardiomyocyte turn-over, as several studies could show that cTn increase during exercise was not associated with any immediate or longer-term functional impairment (25,26).
In all three running groups baseline cTnI values were above the limit of detection (LOD), baseline cTnT values were at the LOD of the assay. This reflects the differences in analytical sensitivity of the 2 cTn assays, which had been previously described (6).
Long term training effect possibly causing heart muscle enlargement could be a possible explanation for the higher baseline cTnI values seen in the trained athletes when compared to control group. This effect was confirmed in another study looking at the consequences of endurance training on cTn values (27-29).
cTn increases were seen in all running groups and levels were in some cases higher than the 99th percentile upper reference value of a normal population in 50% of the marathon and semi-marathon runners. Dynamics of cTn release are essential for diagnosing myocardial infarction in symptomatic chest pain patients and current guidelines indicate that clinical symptoms as well as a rise/fall of cTn are required for the diagnosis of AMI (17,30). This must be always checked when symptomatic patients after exercising are presenting to emergency room with possible diagnosis of myocardial infarction as this could possibly cause difficulties in diagnosis of acute myocardial infarction (31). Even relative low impact 1 hour running in untrained runners could result in cTn values above the 99th percentile upper reference limit.
NPs play an important role in the regulation of cardiac function. Running exercise leads to a small but significant increase of both NPs; BNP and NT-ProBNP in all 3 running groups. It has been described that exercise induced increase of NPs was associated with exercise duration, a fact which is confirmed with this study. The increase in the higher impact running group was more pronounced when compared to control running group. NP increase may be related transient myocardial wall stress, cardiomyocyte metabolic effects or exercise induced neuro endocrine response to the myocardial stretch (32).
Ischemia can trigger inflammatory response, along with macrophage infiltration and fibroblast activation (33). This could also be the explanation for the significant Gal-3 increase in all 3 groups, being higher in the more stringent and longer distance runners. A similar drastic increase of Gal-3 correlating with the intensity and duration of exercise has been described in endurance athletes however with no impact on heart function was shown after further analysis by cardiac magnetic resonance testing (CMR) and 2D and 3D echocardiography to assess left and right ventricular ejection fraction (34).
Exercise induced right ventricular dysfunction and structural remodeling has been described in endurance athletes, however long-term consequences are still not defined (35). Recent results of a contrast-enhanced CV magnetic resonance study have questioned the development of an exercise induced right ventricular cardiomyopathy (26). In the absence of long-term follow-up studies this conclusion should be viewed with caution.
Observed changes for cTn and Gal-3 levels were exceeding the short- and long-term biological variability reported (36). Variability of the individual cTn and Gal-3 response at the different time points in the running groups was also high, with more extreme elevations in some runners. The inter-individual variability with a low index of individuality could be one reason for the different patterns in cTn responses (16,37). However, as described also in other studies, major factors influencing cTn and Gal-3 elevations are duration and intensity of the running exercise (36,37).
NT-ProBNP is the inactive part with longer half-life, which is cleared only by the kidney. BNP is the active hormone with shorter half-life and break down via additional pathways through specific receptors and circulating endogenous peptidases. Direct influence of kidney impairment on NT-ProBNP could be the reason for the stronger association with renal dysfunction observed in patients with heart failure but also in the different running groups after stringent exercise (38).
Gal-3 levels in the different running groups also showed significant correlation to the renal function markers Creatinine and Cystatin C. Independent association of Gal-3 levels with renal function has been described however it is suggested that it is also causally involved in mechanisms of tubulointerstitial fibrosis and CKD progression (39).
Major strength of our study was the compliance to the same protocol for the 3 running groups using same devices for blood drawing, same investigator and parallel testing of the cardiac markers, therefore no bias due to these points.
There were several limitations to this study. The number of athletes in the different running group is quite low due to the difficulties to recruit such individuals especially for the blood test 3h after the run. Nevertheless, our data shows that all three cardiac biomarkers increase in the different running groups, with lower intensity in the control group. There was also no longer-term follow-up of patients, so the increases of the three cardiac biomarkers could not be related to longer term outcomes.
In conclusion, the question whether running exercise of different intensity could be harmful to the heart has no simple answer. We could show that running exercise can be associated with biochemical abnormalities that may reflect adverse consequences on the heart like possible micro necrosis, oxidative stress, fibrosis and myocardial stretch. With exception of Troponin where levels continue to raise after end of running, NPs and Gal-3 levels normalized relatively fast after the exercise proofing that stringent exercise induced heart stretch and induced inflammatory processes may be not sufficient to cause longer term heart remodeling and fibrosis. The possible harmful effect of longer-term cardiac consequences of repeated intensive sport activities still needs to be demonstrated. Short term however exercise induced changes in Troponin levels should be excluded in chest pain patients presenting to the emergency department.
Acknowledgments
ARCHITECT reagents for the study were provided by Abbott Laboratories Wiesbaden.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.01.04). Etienne Cavalier serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2017 to June 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Study was approved by Ethic committee of the University of Liege. All the subjects signed an informed consent (Approval ID: B7007201110897).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paffenbarger RS JR, Wing AL, Hyde RT, et al. Physical activity and incidence of hypertension in college alumni. Am J Epidemiol 1983;117:245-57. [Crossref] [PubMed]
- Albert CM, Mittleman MA, Chae CU, et al. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 2000;343:1355-61. [Crossref] [PubMed]
- Chugh SS, Weiss JB. Sudden cardiac death in the older athlete. J Am Coll Cardiol 2015;65:493-502. [Crossref] [PubMed]
- Baker P, Davies SL, Larkin J, et al. Changes to the cardiac biomarkers of non-elite athletes completing the 2009 London Marathon. Emerg Med J 2014;31:374-9. [Crossref] [PubMed]
- Vilela EM, Bastos JC, Rodrigues RP, et al. High-sensitivity troponin after running--a systematic review. Neth J Med 2014;72:5-9. [PubMed]
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574-81. [Crossref] [PubMed]
- Hubble KM, Fatovich D, Grasko JM, et al. Cardiac troponin increases among marathon runners in the Perth Marathon: the Troponin in Marathons (TRIM) study. Med J Aust 2009;190:91-3. [PubMed]
- Mingels A, Jacobs L, Michielsen E, et al. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem 2009;55:101-8. [Crossref] [PubMed]
- Salvagno GL, Schena F, Gelati M, et al. The concentration of high-sensitivity troponin I, galectin-3 and NT-proBNP substantially increase after a 60-km ultramarathon. Clin Chem Lab Med 2014;52:267-72. [Crossref] [PubMed]
- Gaze DC, Prante C, Dreier J, et al. Analytical evaluation of the automated galectin-3 assay on the Abbott ARCHITECT immunoassay instruments. Clin Chem Lab Med 2014;52:919-26. [Crossref] [PubMed]
- Perk J, De BG, Gohlke H, et al. G Ital Cardiol (Rome) 2013;14:328-92. [European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (version 2012) The Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts)]. [PubMed]
- Krintus M, Kozinski M, Boudry P, et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med 2014;52:1657-65. [PubMed]
- Mair J, Gerda F, Renate H, et al. Head-to-head comparison of B-type natriuretic peptide (BNP) and NT-proBNP in daily clinical practice. Int J Cardiol 2008;124:244-6. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Mongia SK, La’Ulu SL, Apple FS, et al. Performance characteristics of the Architect brain natriuretic peptide (BNP) assay: a two site study. Clin Chim Acta 2008;391:102-5. [Crossref] [PubMed]
- Yeo KT, Wu AH, Apple FS, et al. Multicenter evaluation of the Roche NT-proBNP assay and comparison to the Biosite Triage BNP assay. Clin Chim Acta 2003;338:107-15. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Kardiol Pol 2015;73:1207-94. [Crossref] [PubMed]
- Hrynchyshyn N, Jourdain P, Desnos M, et al. Galectin-3: a new biomarker for the diagnosis, analysis and prognosis of acute and chronic heart failure. Arch Cardiovasc Dis 2013;106:541-6. [Crossref] [PubMed]
- Le Goff C, Kaux JF, Goffaux S, et al. Cardiac biomarkers and cycling race. J Sports Sci Med 2015;14:475-6. [PubMed]
- Goette A, Bukowska A, Dobrev D, et al. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J 2009;30:1411-20. [Crossref] [PubMed]
- Hultman E, Sahlin K. Acid-base balance during exercise. Exerc Sport Sci Rev 1980;8:41-128. [PubMed]
- Shave R, Baggish A, George K, et al. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol 2010;56:169-76. [Crossref] [PubMed]
- Mair J, Lindahl B, Hammarsten O, et al. How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care 2018;7:553-60. [Crossref] [PubMed]
- Hammarsten O, Mair J, Möckel M, et al. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018;23:725-34. [Crossref] [PubMed]
- O’Hanlon R, Wilson M, Wage R, et al. Troponin release following endurance exercise: is inflammation the cause? a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2010;12:38. [Crossref] [PubMed]
- Bohm P, Schneider G, Linneweber L, et al. Right and Left Ventricular Function and Mass in Male Elite Master Athletes: A Controlled Contrast-Enhanced Cardiovascular Magnetic Resonance Study. Circulation 2016;133:1927-35. [Crossref] [PubMed]
- Legaz-Arrese A, Lopez-Laval I, George K, et al. Impact of an endurance training program on exercise-induced cardiac biomarker release. Am J Physiol Heart Circ Physiol 2015;308:H913-20. [Crossref] [PubMed]
- Legaz-Arrese A, George K, Carranza-Garcia LE, et al. The impact of exercise intensity on the release of cardiac biomarkers in marathon runners. Eur J Appl Physiol 2011;111:2961-7. [Crossref] [PubMed]
- Lara B, Salinero JJ, Gallo-Salazar C, et al. Elevation of Cardiac Troponins After Endurance Running Competitions. Circulation 2019;139:709-11. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581-98. [Crossref] [PubMed]
- Eijsvogels TM, Januzzi JL, Taylor BA, et al. Impact of statin use on exercise-induced cardiac troponin elevations. Am J Cardiol 2014;114:624-8. [Crossref] [PubMed]
- Herrmann M, Scharhag J, Micle A M, et al. Post-race kinetics of cardiac troponin T and I and N-terminal pro-brain natriuretic peptide in marathon runners. Clin Chem 2003;49:831-4. [Crossref] [PubMed]
- Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol 2008;130:147-58. [Crossref] [PubMed]
- Hattasch R, Spethmann S, De Boer RA, et al. Galectin-3 increase in endurance athletes. Eur J Prev Cardiol 2014;21:1192-9. [Crossref] [PubMed]
- La GA, Burns AT, Mooney DJ, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 2012;33:998-1006. [Crossref] [PubMed]
- Nordenskjold AM, Ahlstrom H, Eggers KM, et al. Short- and long-term individual variation in cardiac troponin in patients with stable coronary artery disease. Clin Chem 2013;59:401-9. [Crossref] [PubMed]
- Neilan TG, Januzzi JL, Lee-Lewandrowski E, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation 2006;114:2325-33. [Crossref] [PubMed]
- Mccullough PA, Sandberg KR. B-type natriuretic peptide and renal disease. Heart Fail Rev 2003;8:355-8. [Crossref] [PubMed]
- Drechsler C, Delgado G, Wanner C, et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. J Am Soc Nephrol 2015;26:2213-21. [Crossref] [PubMed]
Cite this article as: Le Goff C, Lennartz L, Vranken L, Kaux JF, Cavalier E. Comparison of cardiac biomarker dynamics in marathon, semi-marathon and untrained runners: what is the impact on results interpretation? J Lab Precis Med 2019;4:6.


