
Is one cardiac troponin better than the other?
Introduction
According to the fourth universal definition of myocardial infarction (1), which has been subscribed or endorsed by the vast majority of cardiology associations and organizations worldwide, an ischemic myocardial injury shall now be diagnosed in the presence of a rise and/or a fall of cardiac troponins value, with at least one measurement exceeding the 99th percentile of the upper reference limit (URL), and accompanied by at least: (I) symptoms of myocardial ischemia; (II) newly developed ischemic electrocardiography (ECG) abnormalities (including Q waves); (III) evidence of newly onset loss of viable myocardium or regional wall motion abnormalities in a pattern suggestive for ischemia; and (IV) detection of intracoronary thrombi during angiographic assessment or autopsy.
This latest characterization of acute myocardial infarction (AMI), which appears a rather commonsensical evolution of the previous definitions (2), further emphasizes a central aspect in AMI diagnostics. Essentially, the measurement of cardiac troponins has been reiterated as biochemical gold standard for diagnosing myocardial injuries, thus also including irreversible myocardial ischemia. This conclusion is strongly supported by evidence that the measurement of additional laboratory tests such as creatine kinase isoenzyme MB (CK-MB), myoglobin, copeptin, heart-type fatty acid binding protein (h-FABP) or ischemia modified albumin among others would no longer be cost-effective, since the assessment of these other biomarkers could only provide modest or no incremental clinical information whilst contextually (and unreasonably) enhancing laboratory expenditures (3).
The so-called troponin complex is basically formed by three regulatory proteins, which are integral to skeletal and cardiac muscle contraction. The three protein moieties have been defined according to their basic function in muscle contraction. Therefore, troponin I (TnI) is named for “Inhibiting” the ATP-ase activity of actomyosin, troponin T (TnT) for binding “Tropomyosin” and troponin C (TnC) for binding “Calcium” (4). Importantly, although the biochemical structure of TnC is almost identical in both skeletal and cardiac muscle, TnT and TnI are encoded by specific genes in the cardiac muscle, which hence produce the cardiac-specific isoforms cTnT (troponin T2 cardiac-type gene; TNNT2) and cTnI (troponin I3 cardiac-type gene; TNNI3) (5). Park et al. (6) carried out a protein alignment study and found that human cTnI only displays 63% and 57% homology with slow-twitch skeletal muscle isoform TnI (ssTnI) and fast-twitch skeletal muscle (fsTnI), respectively. A direct comparison of the cardiac and skeletal muscle isoforms of both cTnI and cTnT is provided in Figure 1, as retrieved from the National Center for Biotechnology Information (NCBI) protein structure database (7).
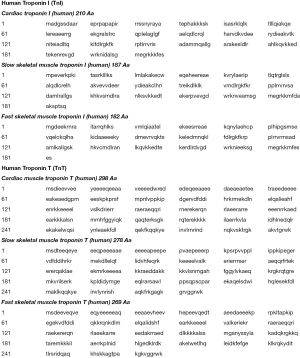
The providential structural heterogeneity of cardiac and skeletal muscle TnT and TnI has then enabled to produce monoclonal antibodies specifically targeting epitopes contained in the cardiac counterparts, which have been successfully used for manufacturing commercial immunoassays and for specifically measuring cardiac troponins in serum or plasma of patients with cardiac injury (8). Although it has been now clearly established that the degradation of the troponin complex after cardiac injuries may induce post-translational modifications that would finally alter antibody binding (9,10), Katrukha et al. recently showed that samples drawn at different time points from different patients with AMI displayed a quite similar array of cTnI fragments, and that monoclonal antibodies specifically targeting the central 34–126 amino acid domain of cTnI were capable to bind all the various immunoreactive isoforms (11). Similar evidence has been provided for cTnT (12), thus suggesting that cardiac troponin degradation occurs at a greater extent in the injured cardiac tissue than in the bloodstream.
The unremitting refinement of cardiac troponin immunoassays since their first appearance at the end of the last century has now allowed to produce and commercialize the so-called high-sensitivity (HS) immunoassays (13), which enable to detect few micrograms of injured myocardial tissue, so allowing to diagnose very modest and even transitory cardiac injuries (14-16). The diagnostic superiority and cost-effectiveness of these innovative methods over the former generation of contemporary-sensitive techniques has now been unquestionably established (17,18).
Despite the role of cardiac troponins for diagnosing myocardial ischemia is hence unquestionable, a dilemma is still engaging the minds of many clinicians and laboratory professionals. Is the measurement of one cardiac troponin (i.e., cTnT or cTnI) better than the other? (19). This substantial question has persuasively emerged during the past decade, and will probably become more relevant in the future as an inevitable consequence of the ongoing process of reorganization and consolidation of laboratory services (20). In this evolving scenario, tenders for purchasing or renting laboratory instrumentation will increasingly broaden, will lead to enhanced consolidation of clinical chemistry and immunochemistry testing, but will also expand beyond the physical boundaries of local laboratories, extending to many diagnostic services within provincial, regional and even national networks (21). This would finally obligate both laboratory professionals and clinicians to submissively implement cardiac troponin immunoassays (either cTnT or cTnI), whose procurement will be included in large tenders for supplying laboratory instrumentation and reagents. Since finding a reliable answer to the question as to whether one cardiac troponin is better than the other would strongly condition our practical behaviors, we will try to solve the puzzle in the following part of this article, with the help of some evidence-based information.
Clinical evidence
The number of studies which have assessed the diagnostic performance of cardiac troponins for diagnosing AMI is colossal. A simple Medline (PubMed interface) search using the keywords “troponin” AND “myocardial infarction” produces now over 7,800 hits. To restrict our search, we have arbitrarily decided to select the largest studies published so far, containing simultaneous information on at least 3 different HS cardiac troponin immunoassays, with diagnostic performance calculated on admission and 1, 2 and 3 hours afterward, and including ≥300 patients (Table 1).
Table 1
| Authors (reference) | Patients | At admission, AUC | Serial sampling | ||
|---|---|---|---|---|---|
| 1-h AUC | 2-h AUC | 3-h AUC | |||
| Reichlin et al., 2009 (22) | 718 (123 with AMI; 17%) | Roche cTnT: 0.96; |
Roche cTnT: 0.98; |
Roche cTnT: 0.96; |
Roche cTnT: 0.98; |
| Reiter et al., 2011 (23) | 401 aged >70 years (98 with AMI; 24.4%) | Roche cTnT: 0.92; |
Roche cTnT: 0.95; |
Roche cTnT: 0.96; |
Roche cTnT: 0.97; |
| Boeddinghaus et al., 2019 (24) | 1,579 (243 with AMI; 15.4%) | Roche cTnT: 0.94; |
Roche cTnT: 0.96; |
Roche cTnT: 0.97; |
Roche cTnT: 0.97; |
| Twerenbold et al., 2015 (25) | 447 with renal dysfunction (160 with AMI; 35.8%) | Roche cTnT: 0.87; |
Roche cTnT: 0.90; |
Roche cTnT: 0.91; |
Roche cTnT: 0.94; |
AUC, area under the curve; AMI, acute myocardial infarction; cTnI, cardiac troponin I; cTnT, cardiac troponin T.
The very first study based on the new HS cardiac troponin immunoassays was published by Reichlin et al., in 2009 (22). The authors used two HS cTnI and one HS cTnT immunoassays for studying 718 consecutive patients admitted to the emergency department with signs or symptoms of acute coronary syndrome, 123 (17%) of whom were finally diagnosed has having an AMI. The diagnostic performance of the different immunoassays at the different time points was almost overlapping, with an area under the curve (AUC) comprised between 0.96 and 0.98 (Table 1).
In an ensuing study, Reiter et al used two HS cTnI and one HS cTnT immunoassays in 401 consecutive patients aged >70 years, admitted to the ED with signs or symptoms suggestive for acute coronary syndrome, in 98 (24.4%) of whom a final diagnosis of AMI could be adjudicated (23). Even in this study the diagnostic performance of the different immunoassays at different time points was very similar, displaying an AUC comprised between 0.92 and 0.97 (Table 1).
More recently, Boeddinghaus et al. used two HS cTnI and one HS cTnT immunoassays in 1,579 patients consecutively admitted to the emergency department with signs or symptoms of acute coronary syndrome, 243 (15.4%) of whom were finally diagnosed has having an AMI (24). Like previous findings, the diagnostic performance of the three different immunoassays at the different time points was very similar, with an AUC ranging between 0.92 and 0.98 (Table 1).
Additional useful information could then be garnered from some other investigations, with different populations or study design. Twerenbold et al. performed a multicenter study, based on the use of 4 HS cardiac troponin immunoassays in 2,813 patients admitted with symptoms of AMI, 447 (i.e., 16%) with impaired renal function (25). A final diagnosis of AMI was made in 160 (i.e., 35.8%) patients with renal dysfunction. Interestingly, the overall diagnostic performance of the four different cardiac HS troponin immunoassays (one HS cTnT and three HS cTnI methods) not only was extremely similar in the entire cohort of patients (all AUCs comprised between 0.93 and 0.94), but was also virtually identical in the subset of patients with impaired renal function (Table 1). Another interesting study was recently published by Schaaf et al., who studied the kinetics of cardiac troponins in 29 consecutive patients diagnosed with ST-elevation myocardial infarction (STEMI) (26). Cardiac troponins were measured with three different HS immunoassays and the course of myocardial injury was monitored with contrast-enhanced cardiac magnetic resonance. Interestingly, the correlation between infarct mass and peak troponin value was 0.73 for Roche HS cTnT, 0.69 for Abbott HS cTnI and 0.57 for Siemens HS cTnI, respectively, whilst the AUC of cardiac troponin peak value for detecting microvascular obstruction was virtually identical (Roche HS cTnT, 0.91; Abbott HS cTnI, 0.98; Siemens HS cTnI, 0.86).
Conclusions
The current diagnostic armamentarium of cardiac troponin immunoassays is relatively vast. Regular updates on the characteristics of the different methods can be found in the website of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) (27). Due to a licensing agreement, the cTnT HS immunoassay is only marketed by Roche Diagnostics, whilst other in vitro diagnostic (IVD) companies have developed and commercialized cTnI HS methods. It is undeniable that a substantial variability still makes the results of currently available cardiac troponin immunoassays poorly comparable, so that the standardization of HS methods remains an unmet target, not only between cTnI and cTnT techniques, but also among the various cTnI immunoassays (28). Nevertheless, published evidence suggests that the diagnostic performance of the currently available HS techniques seems highly comparable. Overall, the diagnostic efficiency at the different time points has been found nearly overlapping, irrespective of renal function, in the four large studies that we have reviewed (Table 1). Just minor differences were observed, which are not likely to influence the diagnostic performance of these immunoassays in clinical practice, as shown in Figure 2 and Table 2 (P always >0.5 for multiple comparisons).

Table 2
| Immunoassay | n | Mean | 95% CI |
|---|---|---|---|
| Roche cTnT | 13 | 0.958 | 0.948–0.969 |
| Abbott cTnI | 13 | 0.952 | 0.941–0.963 |
| Siemens cTnI | 9 | 0.959 | 0.948–0.969 |
| Beckman Coulter cTnI | 5 | 0.958 | 0.940–0.976 |
95% CI, 95% confidence interval; cTnI, cardiac troponin I; cTnT, cardiac troponin T.
Therefore, both clinicians and laboratory professionals should be reassured that the currently available and clinically validated commercial cardiac troponin immunoassays perform equally in diagnosing AMI, regardless of time of patient admission and renal function. According to currently reliable evidence, it seems hence reasonable to conclude that one cardiac troponin is not better than the other for diagnosing AMI, neither one HS cTnI commercial immunoassay seems to exhibit better diagnostic performances compared to the others.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.04.06). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231-64. [Crossref] [PubMed]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
- Lippi G, Plebani M. Understanding cardiac troponin biology: all other cardiac biomarkers shall rest in peace? J Lab Prec Med 2019;4:9. [Crossref]
- Bucher EA, Maisonpierre PC, Konieczny SF, et al. Expression of the troponin complex genes: transcriptional coactivation during myoblast differentiation and independent control in heart and skeletal muscles. Mol Cell Biol 1988;8:4134-42. [Crossref] [PubMed]
- Lippi G, Cervellin G. Genetic polymorphisms of human cardiac troponins as an unrecognized challenge for diagnosing myocardial injury. Int J Cardiol 2014;171:467-70. [Crossref] [PubMed]
- Park KC, Gaze DC, Collinson PO, et al. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017;113:1708-18. [Crossref] [PubMed]
- National Center for Biotechnology Information. Protein database. Available online: https://www.ncbi.nlm.nih.gov/protein/. Last access, 4 April, 2019.
- Sharma S, Jackson PG, Makan J. Cardiac troponins. J Clin Pathol 2004;57:1025-6. [Crossref] [PubMed]
- Lippi G, Cervellin G. Degradation of troponin I in serum or plasma: mechanisms, and analytical and clinical implications. Semin Thromb Hemost 2012;38:222-9. [Crossref] [PubMed]
- Mair J. What is new on cardiac troponin degradation? J Lab Precis Med 2017;2:55. [Crossref]
- Katrukha IA, Kogan AE, Vylegzhanina AV, et al. Full-Size Cardiac Troponin I and Its Proteolytic Fragments in Blood of Patients with Acute Myocardial Infarction: Antibody Selection for Assay Development. Clin Chem 2018;64:1104-12. [Crossref] [PubMed]
- Streng AS, de Boer D, van Doorn WP, et al. Identification and Characterization of Cardiac Troponin T Fragments in Serum of Patients Suffering from Acute Myocardial Infarction. Clin Chem 2017;63:563-72. [Crossref] [PubMed]
- Galli C, Lippi G. High-sensitivity cardiac troponin testing in routine practice: economic and organizational advantages. Ann Transl Med 2016;4:257. [Crossref] [PubMed]
- Lippi G, Cervellin G, Schena F. How much myocardium mass may be injured during endurance physical exercise? Clin Chim Acta 2017;470:29-30. [Crossref] [PubMed]
- Marjot J, Kaier TE, Martin ED, et al. Quantifying the Release of Biomarkers of Myocardial Necrosis from Cardiac Myocytes and Intact Myocardium. Clin Chem 2017;63:990-6. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F. "Ultra-sensitive" cardiac troponins: Requirements for effective implementation in clinical practice. Biochem Med (Zagreb) 2018;28:030501 [Crossref] [PubMed]
- Lippi G, Bonfanti L, Dipalo M, et al. Clinical, organizational and economic analysis of high-sensitivity cardiac troponin testing in the emergency department. Ann Res Hosp 2017;1:44. [Crossref]
- Clerico A, Lippi G. The state-of-the-art of “high-sensitivity” immunoassay for measuring cardiac troponin I and T. J Lab Precis Med 2018;3:53. [Crossref]
- Giuliani S, Dieplinger B, Mueller T. Head-to-head comparison of three different high-sensitivity cardiac troponin assays for early rule-in and rule-out of acute myocardial infarction. J Lab Precis Med 2019;4:4. [Crossref]
- Lippi G, Mattiuzzi C. Project Management in Laboratory Medicine. J Med Biochem 2019; In Press. [Crossref]
- Lippi G, Cervellin G. Choosing troponin immunoassays in a world of limited resources. J Am Coll Cardiol 2013;62:647-8. [Crossref] [PubMed]
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858-67. [Crossref] [PubMed]
- Reiter M, Twerenbold R, Reichlin T, et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J 2011;32:1379-89. [Crossref] [PubMed]
- Boeddinghaus J, Nestelberger T, Twerenbold R, et al. High-Sensitivity Cardiac Troponin I Assay for Early Diagnosis of Acute Myocardial Infarction. Clin Chem 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Twerenbold R, Wildi K, Jaeger C, et al. Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients With Renal Dysfunction. Circulation 2015;131:2041-50. [Crossref] [PubMed]
- Schaaf M, Huet F, Akodad M, et al. Which high-sensitivity troponin variable best characterizes infarct size and microvascular obstruction? Arch Cardiovasc Dis 2019; [Epub ahead of print]. [Crossref] [PubMed]
- International Federation of Clinical Chemistry and Laboratory Medicine. Task Force on Clinical Applications of Cardiac Bio-Markers. Available online: http://www.ifcc.org/media/463453/HighSensitivityCardiacTroponinI_T_AssayAnalyticalCharacteristics_v060617.pdf. Last access, April 4th, 2019.
- Christenson RH, Jacobs E, Uettwiller-Geiger D, et al. Comparison of 13 Commercially Available Cardiac Troponin Assays in a Multicenter North American Study. J Appl Lab Med 2017;1:544-61. [Crossref]
Cite this article as: Lippi G, Cervellin G. Is one cardiac troponin better than the other? J Lab Precis Med 2019;4:19.


