
B-type natriuretic peptide in the Emergency Department: the impact of restricted policies on requesting patterns and cost
Introduction
Plasma concentrations of natriuretic peptides are helpful in the diagnosis of heart failure (HF) as a low-normal concentration in an untreated patient makes HF unlikely as the cause of symptoms (1) and should obviate the need for further cardiac tests such as echocardiography as well as more expensive investigations (2). Indeed, in clinical practice, the use of B-type natriuretic peptides (BNPs) is as a “rule out’ test to exclude significant cardiac disease particularly in primary care, but also in certain settings of secondary care as the Emergency Department (ED) and Clinics (3). Since 2005, the European Society of Cardiology (ESC) recommends excluding suspected HF in symptomatic patients through electrocardiogram and measurement of BNP (3).
However an international survey revealed that in 2013, 20% of surveyed laboratories did not offer this test in their menu, showing that this type of service was not yet universal since the test was not available in all laboratories (4). There was a moderate increase (12%) of laboratories measuring BNP (4) compared to the initial survey in 2006 (5). The implementation of BNP measurement for HF management was a slow process between 2006 and 2009 at a time when guidelines had just been established (4). In the institutions where it is available, there are marked differences in the requesting pattern, with a 297-fold variation in requests (6).
Recent 2016 ESC guidelines for the diagnosis and treatment of acute and chronic HF recommend to start the diagnostic work-up in the pre-hospital setting and continue in the ED in order to establish the diagnosis in a timely manner and initiate appropriate management (1,3). However the optimal ordering for BNP, as in any laboratory test, must be achieved. A recent study showed an over-requesting of BNP in the ED, and proposed a successful corrective measure through algorithms supported by the medical direction, transmission and dissemination of information and knowledge, and awareness of the cost of the tests by the doctors (7).
A prior REDCONLAB group study that focused on the use of stat laboratory tests from ED, by evaluating geographical differences in requesting patterns reported 67% of the participant laboratories had BNP available for request from ED, and that the overall demand was 14.52 per 1,000 ED patient’s admissions (8).
The aim of this research was to study the requesting pattern of BNP in the ED, the expenses associated with it and the effects of restricted criteria for its measurement.
Methods
Setting
The Spanish National Health System is divided in every region into Health Departments (HDs). Each HD covers a geographic area and usually has a unique Hospital that serves in-, out- and ED patients. The laboratory located at the Hospital attends the needs of every HD inhabitant, including ED patient’s stat laboratory tests, that are needed immediately in order to manage medical emergencies.
Data collection
The questionnaire was addressed to the 110 participants of the previous REDCONLAB study (9), requesting the number of BNP ordered by ED clinicians, number of patients admitted in the ED for the year 2016, and for the reagent cost of BNP. Laboratories where BNP was available for request in the ED were divided in two groups; those where BNP could be freely ordered requested in the ED (free availability group), and those where it was regulated by algorithms/internal policies (restricted group).
Data processing
After collecting the data, a test-utilization rate was calculated by standardizing the number of requests by the number of ED admissions; it was expressed as number of BNP tests per 1,000 ED admissions (BNP/1,000 ED admissions). The rate was compared between laboratories with restricted and with free availability of BNP.
We calculated how many BNP tests would have not been ordered if laboratories where the request was not regulated would have had the same as the ones where it was. We finally calculated the potential economic savings.
Statistical analysis
All analyses were performed using SPSS Inc. for Windows, Version 21.0. (Chicago, SPSS Inc.). Descriptive statistics were generated for test-utilization rates. The analysis of the distribution of the number of test requests per 1,000 ED admissions was conducted using the Kolmogorov-Smirnoff test.
The difference in BNP/1,000 ED admissions between restricted-BNP group and availability BNP group was assessed using a Mann-Whitney statistic.
A two-sided P≤0.05 rule was utilized as the criterion for rejecting the null hypothesis of no difference.
Results
Out of the 110 invited laboratories, 65 (59%) participated in the study.
The overall median number of ED admissions during year 2016 was 70,486 (IQR, 40,598.5–140,105.5). In 21 (32.3%) laboratories, BNP was not available for request in the ED. The median rate of request of BNP per 1,000 ED admissions in the remaining 44 laboratories was 32.2 (IQR: 47.6), with a significant variability among participant institutions.
Twenty-three (52.3%) laboratories conformed the free availability and 21 (48%) the restricted group.
The request of BNP was significantly lower in laboratories belonging to the restricted availability group, as opposed to the ones in the free-availability cohort (47.9 vs. 21.4, P=0.013) (Figure 1).
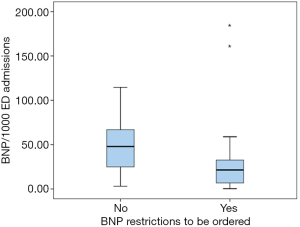
Thirty-eight laboratories reported the price of the reagent; the average price per test was 11.7€. Taking into account that cost, 1,665,833€ were spent in one year, to measure a total of 154,702 BNP tests requested from ED that attended a total of 6,478,575 medical admissions in one year.
A total of 42,962 BNP tests could have been not measured in 2016 if the request in the free availability group would have been the same as in the restricted counterpart, with potential savings of 462,615€, only in reagent costs.
Discussion
One third of participating laboratories still did not offer BNP in the ED, despite current recommendations, and the fact that BNP is universally accepted as tool for the etiologic diagnosis of dyspnoea in acutely ill patients (10) suggesting that there is need to spread the availability of this biomarker. The overall request is higher than previous studies (8), however is lower in EDs with a restricted policy regarding the test demand.
The potential of a biomarker to impact the diagnosis/management of a particular disorder depends on to how it is used. It is a priority to align the request with the clinical indication or patient clinical question. Independently of the clear evidence regarding the use of BNP in ED clinical practice, the tests cannot be offered without a clear guidance or protocol on its use (7). However more than 1.5 million € were spent during 2016 on reagent to measure 154,702 BNP in EDs attending 6,478,575 medical admissions. There was a significant variability in the request of BNP, which was significantly lower in institutions with policies/algorithms regulating its request. Should the request in the institutions with no such regulation would approximate their counterparts, more than €400,000 would have been saved, only in reagent costs. Our research shows that 48% of laboratories had policies for an adequate request of BNP. It also shows that protocols for BNP demand contention works, as laboratories that had such policies had a lower demand.
As a whole, our results show that there was likely an overall BNP under-request. In fact there are ED clinicians in certain institutions that cannot order a BNP to find out the etiologic diagnosis of patients presenting with dyspnea. However, there is likely a potential over request in certain laboratories in the free availability group; as there were significant differences when compared to the restricted group. Indeed, if the laboratories without policies for an adequate BNP request would approach the less demanding, 42,962 tests would have been not requested with economic savings of 462,615€.
The relatively high cost of BNP, the potential adverse effects of both an under- and over- request, and the fact that still one third of laboratories do not even offer it in the ED, suggest there is a need to improve this process, through interventions designed and established in consensus between the clinical laboratories and the ED clinicians.
Limitations
The study had certain limitations. First, the differences in BNP request between ED could be explained by the voluntary participation in the study that could generate a potential selection bias, and, although confirmed, we do not know the reasons of the two outliers. Second, we do not know the reason for not offering ProBNP for its request, since it would be interesting to know if because lack of resources or technology. We also do not know if ProBNP was appropriately requested in the restricted BNP group or inappropriately in the free availability group since we could not review every medical record to verify if the clinical suspicion supported the request. Finally, the calculated economic savings have only been calculated on the basis of the reagent price without considering other costs such as staff, instruments, maintenance and may not apply to other countries or settings, since our laboratories belong to the Public Health Network, where reagent prices are very low.
Conclusions
More than 1.5 million € were spent on reagent to measure BNP in EDs attending 6,478,575 medical admissions in one year. One third of laboratories did not offer BNP in the ED, despite current recommendations. Institutions with policies regulating the request if the test had a lower rate of requests that those with no such regulations. If the latter would have similar algorithms, more than €400,000 could have been saved, only in reagent costs.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161-7. [Crossref] [PubMed]
- Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005;26:1115-40. [Crossref] [PubMed]
- Hammerer-Lercher A, Collinson P, van Dieijen-Visser MP, et al. Do laboratories follow heart failure recommendations and guidelines and did we improve? The CARdiac MArker Guideline Uptake in Europe (CARMAGUE). Clin Chem Lab Med 2013;51:1301-6. [Crossref] [PubMed]
- Pulkki K, Suvisaari J, Collinson P, et al. A pilot survey of the use and implementation of cardiac markers in acute coronary syndrome and heart failure across Europe The CARdiac MArker Guideline Uptake in Europe (CARMAGUE) study. Clin Chem Lab Med 2009;47:227-34. [Crossref] [PubMed]
- Public Health England. The NHS atlas variation in diagnostic services : reducing unwarranted variation to increase value and improve quality [Internet]. NHS. 2013. Available online: http://fingertips.phe.org.uk/profile/atlas-of-variation
- Ray P, Delerme S, Jourdain P, et al. Differential diagnosis of acute dyspnea: the value of B natriuretic peptides in the emergency department. QJM 2008;101:831-43. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Uris J, et al. Variation in laboratory tests ordered for patients treated in hospital emergency departments. Emergencias 2014;26:450-8.
- Salinas M, López-Garrigós M, Flores E, et al. Big differences in primary care celiac disease serological markers request in Spain. Biochem Med (Zagreb) 2017;27:231-6. [Crossref] [PubMed]
- Berdagué P, Caffin PY, Barazer I, et al. Use of N-terminal prohormone brain natriuretic peptide assay for etiologic diagnosis of acute dyspnea in elderly patients. Am Heart J 2006;151:690-8. [Crossref] [PubMed]
Cite this article as: Salinas M, López-Garrigós M, Flores E, Ahumada M, Leiva-Salinas C. B-type natriuretic peptide in the Emergency Department: the impact of restricted policies on requesting patterns and cost. J Lab Precis Med 2019;4:20.


