
Analytical evaluation of Radiometer ABL90 FLEX PLUS enzymatic creatinine assay
Introduction
Chronic kidney disease (CKD) has become a worldwide health issue and is associated with considerable clinical, social and economic consequences. According to recent statistics, the mean prevalence of CKD is approximately 13.4% around the world, with a frequency of patients in stages 3–5 as high as 8.1%, thus accounting for approximately 624 million people suffering from some advanced forms of renal dysfunction around the world (1). Regarding acute kidney injury (AKI), recent epidemiological data suggests that this life-threatening disorder may complicate up to 16% of hospital admissions, resulting in a nearly 4-fold higher risk of in-hospital mortality (2). This concerning epidemiologic information highlights the pivotal importance of an early diagnosis of both CKD and AKI, which would allow more timely and appropriate patient management, so reversing the otherwise unfavourable clinical trend which characterizes acute or chronic renal failure.
The diagnosis of both CKD and AKI strongly relies on laboratory diagnostics (3-5). More specifically, the Kidney Disease Improving Global Outcomes (KDIGO) Update Work Group currently recommends that CKD shall be diagnosed and classified according to glomerular filtration rate (GFR) categories (6). More specifically, stages (from 1 to 5) are defined according to GFR values of ≥90, 89–60, 59–30, 29–15 and <15 mL/min/1.73 m2, respectively. A specific GFR estimating equation is then suggested for initial patient assessment, preferably the 2009 CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine-based formula, according to which creatinine shall be measured in serum or plasma using methods with traceable calibration to the international standard reference materials and minimum bias compared with isotope-dilution mass spectrometry (IDMS) reference methodology (i.e., by using enzymatic assays). As regards AKI, the KDIGO also recommends that this condition shall also be diagnosed and staged according to serum or plasma creatinine values (7). More specifically, a diagnosis of AKI can be made when serum or plasma creatinine increases by ≥26.5 µmol/L within 48 hours or by 1.5-fold during the past 7 days. AKI stages are then also defined according to creatinine variation, whereby an increase between 1.5–1.9 fold or ≥26.5 µmol/L than the baseline defines stage 1, an increase between 2.0–2.9 times than the baseline defines stage 2, whereas an increase >3 fold or ≥353.6 µmol/L than the baseline characterizes stage 3, respectively.
As previously discussed, diagnosing and staging both CKD and AKI strongly rely on accurate and reproducible measurements of serum creatinine. Although the many advantages that have been made in central laboratory assessment of serum creatinine have now allowed to reach a satisfactory degree of accuracy and precision in this measurement, quantification of creatinine with point of care testing (POCT) devices remains problematic, as recently highlighted by Bargnoux et al. (8). In particular, analytical performance goals are still lacking for POCT devices, whilst it may be preferable that their analytical performance would be the same as that of clinical chemistry analyzers. This is an obvious consequence of evidence that the clinical decision making for patients with either CKD or AKI is now virtually identical regardless of whether serum or plasma creatinine is measured with laboratory instrumentation or with POCT devices.
Therefore, this work was aimed at carrying out a comprehensive evaluation of analytical performance of the recently introduced creatinine enzymatic assay on Radiometer ABL90 FLEX PLUS.
Methods
Instrument description
Radiometer ABL90 FLEX PLUS (Radiometer, Copenaghen, Denmark) is a blood gas analyzer designed for POCT in busy clinical environments such as emergency departments (EDs) and intensive care units (ICUs), as well as for being used in physicians’ offices, where obtaining timely and accurate laboratory data may allow rapid diagnostics and more appropriate patient management. The analyzer can perform a range of critical care parameters, including blood gases, electrolytes, co-oximetry and metabolites, including (urea and) creatinine and automatic estimation of GFR (eGFR). As many as 19 parameters can be simultaneously analyzed, using 65 µL of blood, with first results available in 35 seconds. The instrument also enables easy operation by means of automatic sampler aspiration, disposable reagent cartridges, full compatibility with laboratory information system (LIS), integrated automated calibration and quality control usage. The measurement of creatinine on Radiometer ABL90 FLEX PLUS can be carried out in heparinized whole blood with an amperometric enzymatic two-sensor technology. The calibration of this method is traceable to IDMS.
Study protocol
The analytical evaluation of the enzymatic creatinine assay available on Radiometer ABL90 FLEX PLUS encompassed the assessment of both intra-assay, inter-assay and total imprecision, linearity (recovery) and method comparison with a reference enzymatic creatinine assay and a conventional Jaffe technique on a routine clinical chemistry analyzer.
Imprecision studies were carried out by preparing 3 pools of lithium-heparin plasma samples displaying low, normal and high creatinine values (Table 1), which were divided in different aliquots and tested (I) in 20 consecutive runs, during the same analytical session, for estimating intra-assay imprecision; and (II) in duplicate for 10 consecutive working days for calculating the inter-assay imprecision (in such case, aliquots were kept stored at −80 °C until thawing). The linearity of Radiometer ABL90 FLEX PLUS creatinine enzymatic assay was tested by serially diluting a pool of lithium-heparin plasma displaying a high creatinine value (i.e., 445 µmol/L), with a pool of lithium-heparin plasma displaying a low creatinine value (i.e., 47 µmol/L). Accuracy was finally estimated by comparing creatinine values in 130 consecutive routine heparinized blood samples (mean age, 66±16 years; 56 women and 74 men, with a range of creatinine concentrations between 47–445 µmol/L) measured with Radiometer ABL90 FLEX PLUS creatinine enzymatic sensor, and in lithium-heparin plasma obtained after sample centrifugation (at 1,500 ×g for 10 min at room temperature), in which creatinine was assayed with Roche Cobas c702 enzymatic assay and Roche Cobas c702 colorimetric Jaffe compensated assay (Roche Diagnostics, Basel, Switzerland). Both Roche creatinine methods are standardized against IDMS reference methodology. The intra-assay and inter-assay imprecision is 1.2–1.3% and 2.2–2.5% for Roche Cobas c702 colorimetric Jaffe compensated assay, whilst it is 1.3–2.9% and 0.60–1.87% for Roche Cobas c702 enzymatic assay, respectively.
Table 1
| Pools | Intra-assay | Inter-assay | Total | ||||
|---|---|---|---|---|---|---|---|
| Value | CV (%) | Value | CV (%) | CV (%) | |||
| Pool low (μmol/L) | 50.7±0.5 | 0.90 | 51.6±0.9 | 1.78 | 1.99 | ||
| Pool medium (μmol/L) | 98.2±1.2 | 1.26 | 99.3±0.6 | 0.64 | 1.41 | ||
| Pool high (μmol/L) | 287.9±0.9 | 0.33 | 286.6±2.1 | 0.72 | 0.79 | ||
CV, coefficient of variation.
Statistical analysis and Ethical Committee approval
Inter-assay, intra-assay and total imprecision were expressed as coefficient of variation (CV%). Total imprecision was estimated according to the equation of Krouwer and Rabinowitz (9). Linearity was calculated with Pearson’s correlation coefficient, whilst results of method comparison were analyzed with Spearman’s correlation and Bland and Altman plot analysis. The agreement at the five CKD diagnostic thresholds was calculated with Kappa statistics. The statistical analysis was carried out with Analyse-it (Analyse-it Software Ltd., Leeds, UK) and the statistical significance was set at P<0.05. The study was carried out employing pre-existing samples for which routine creatinine assessment was already requested, so that patients’ informed consent was unnecessary. Data obtained on Radiometer ABL90 FLEX PLUS were not reported and did not affect the clinical management of patients. The study was approved by the Ethical Committee of the University Hospital of Verona (SOPAV2; July 25, 2016).
Results
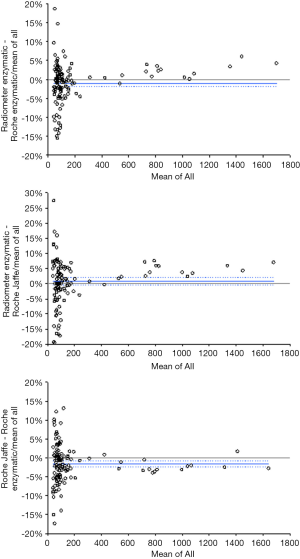
The results of intra-assay, inter-assay and total imprecision are shown in Table 1. Briefly, intra-assay imprecision was between 0.33–1.26%, inter-assay imprecision between 0.64–1.78%, thus generating a total imprecision between 0.79–1.99%. The linearity of the assay, as calculated with Pearson’s correlation, was excellent (r=1.000; P<0.001) in a range of plasma creatinine concentrations between 47 and 445 µmol/L. The results of the method comparison are shown in Figure 1, which allowed calculating the bias between Radiometer ABL90 FLEX PLUS creatinine enzymatic assay and both the Roche Cobas c702 creatinine assays, as well as between the two Roche Cobas c702 creatinine methods. Overall, an excellent correlation was found between creatinine measured on heparinized whole blood with Radiometer ABL90 FLEX PLUS enzymatic assay and plasma creatinine assayed on the same centrifuged samples using Roche Cobas c702 creatinine enzymatic assay (r=0.99; 95% CI, 0.98–0.99; P<0.001) and Roche Cobas c702 creatinine Jaffe assay (r=0.98; 95% CI, 0.98–0.99; P<0.001). The correlation between the two Roche Cobas c702 creatinine methods was also excellent (r=0.99; 95% CI, 0.99–0.99; P<0.001). The percent bias between Radiometer ABL90 FLEX PLUS creatinine enzymatic assay and Roche Cobas c702 creatinine enzymatic assay was −1.0% (95% CI, −1.9% to −0.1%), that with Roche Cobas c702 Jaffe assay was 0.6% (95% CI, −0.6% to 1.9%), whilst that between the two Roche techniques was −1.6% (95% CI, −2.5% to −0.8%). The agreement at the five CKD diagnostic thresholds was 94% (kappa statistics, 0.90; 95% CI, 0.83–0.97; P<0.001) between Radiometer ABL90 FLEX PLUS creatinine enzymatic assay and Roche Cobas c702 creatinine enzymatic assay, 95% (kappa statistics, 0.91; 95% CI, 0.85–0.98; P<0.001) between Radiometer ABL90 FLEX PLUS creatinine enzymatic assay and Roche Cobas c702 Jaffe assay, and 95% (kappa statistics, 0.91; 95% CI, 0.85–0.98; P<0.001) between the two Roche Cobas techniques.
Discussion
Due to an ongoing reorganization of laboratory services worldwide, strongly based on development of networks of clinical laboratories as well as on consolidation of some diagnostic areas and decentralization of others, the use of POCT devices has exponentially grown in the past decades (10,11). This instrumentation has hence become commonplace in short stay and critical care units for producing rapid results, as well as in peripheral laboratories or physician’s offices, where performance of a discrete and appropriate number of diagnostics tests may allow rapid decision making without the need to refer patients to large and frequently overcrowded facilities. Among the various tests that may be useful in these clinical settings, a rapid and accurate assessment of creatinine values would be valuable for screening CKD, for making an early diagnosis of AKI, as well as for assessing the risk of contrast-induced nephropathy and for short- or long-term monitoring of kidney function in the general population as well as in hemodialysed patients (8). Overall, creatinine values would enable taking many life-saving diagnostic decisions, provided that the accuracy of these measurements can be assured.
The results of our imprecision studies revealed that the intra-assay, inter-assay and total imprecision of Radiometer ABL90 FLEX PLUS creatinine enzymatic assay are systematically lower than 2% (Table 1). These results are extremely valid considering that the analytical goals of serum creatinine measured with routine laboratory instrumentation has been set at <8% for diagnosing and staging CKD, and at <4% for diagnosing AKI, respectively (8). Even comparing these data with European Biological Variation Study (EuBIVAS), total imprecision of Radiometer ABL90 FLEX PLUS creatinine enzymatic assay remains lower than desirable imprecision (i.e., 2.2%), bias (i.e., 2.8%) and total error (i.e., 6.4%) for routine enzymatic techniques (12). Notably, the intra-assay imprecision of this POCT method was found to be even lower than that of many commercially available clinical chemistry techniques, including both Roche Cobas methods (13), and was much better than that of many other POCT assays available in the market (14). This would confirm that Radiometer ABL90 FLEX PLUS creatinine enzymatic assay is highly suitable for longitudinal monitoring of blood creatinine. The linearity of the assay was also found to be excellent, between 47–445 µmol/L (i.e., between 0.5–5.0 mg/dL), and thereby suitable for diagnosing kidney impairment throughout a wide measuring range, which would cover the vast majority of clinical samples. Finally, the results of correlation studies with a well-validated routine creatinine enzymatic assay confirm the accuracy of creatinine measurement on Radiometer ABL90 FLEX PLUS. The bias with both the Roche enzymatic and Jaffe methods was ≤1%, thus lower than the 2.2% desirable imprecision cut-off (12), whereas the agreement at CKD diagnostic thresholds was ≥94%.
Conclusions
The results of this comprehensive analytical evaluation of Radiometer ABL90 FLEX PLUS creatinine enzymatic assay confirm that this method is at least as suitable as conventional clinical chemistry enzymatic techniques for routine and urgent diagnosis of kidney diseases.
Acknowledgments
The manufacturer provided in-kind support in the form of equipment and consumables to enable study performance. The manufacturer had no role in directing the study or influencing the interpretation of the study results.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.07.01). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the University Hospital of Verona (SOPAV2; July 25, 2016). The study was carried out employing pre-existing samples for which routine creatinine assessment was already requested, so that patients’ informed consent was unnecessary.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hill NR, Fatoba ST, Oke JL, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0158765 [Crossref] [PubMed]
- Sawhney S, Fraser SD. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Adv Chronic Kidney Dis 2017;24:194-204. [Crossref] [PubMed]
- Delanaye P. Nephrology and clinical chemistry: friends for life! J Lab Precis Med 2018;3:83. [Crossref]
- Makris K. The role of the clinical laboratory in the detection and monitoring of acute kidney injury. J Lab Precis Med 2018;3:69. [Crossref]
- Ebert N, Schaeffner E. New biomarkers for estimating glomerular filtration rate. J Lab Precis Med 2018;3:75. [Crossref]
- Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825-30. [Crossref] [PubMed]
- Kellum JA, Lameire NKDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- Bargnoux AS, Kuster N, Cavalier E, et al. Serum creatinine: advantages and pitfalls. J Lab Precis Med 2018;3:71. [Crossref]
- Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30:290-2. [PubMed]
- Lippi G, Plebani M, Favaloro EJ. The changing face of hemostasis testing in modern laboratories: consolidation, automation, and beyond. Semin Thromb Hemost. 2015;41:294-9. [Crossref] [PubMed]
- Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7. [Crossref]
- Carobene A, Marino I, Coşkun A, et al. The EuBIVAS Project: Within- and Between-Subject Biological Variation Data for Serum Creatinine Using Enzymatic and Alkaline Picrate Methods and Implications for Monitoring. Clin Chem 2017;63:1527-36. [Crossref] [PubMed]
- Hoste L, Deiteren K, Pottel H, et al. Routine serum creatinine measurements: how well do we perform? BMC Nephrol 2015;16:21. [Crossref] [PubMed]
- Shephard MD. Point-of-Care Testing and Creatinine Measurement. Clin Biochem Rev 2011;32:109-14. [PubMed]
Cite this article as: Salvagno GL, Pucci M, Demonte D, Gelati M, Lippi G. Analytical evaluation of Radiometer ABL90 FLEX PLUS enzymatic creatinine assay. J Lab Precis Med 2019;4:26.


