
Alanine aminotransferase—a marker of cardiovascular risk at high and low activity levels
Introduction
Aminotransferases (also called transaminases) are ubiquitous enzymes that catalyze reversible transfer of amino group from amino acids to α-keto acids playing a key role in the metabolism of amino acids in all species. The transamination reaction was discovered in muscle tissue in 1937 by Braunstein and Kritzmann (1) who initially named the enzymes as aminopherases. In 1951 Cammarata and Cohen (2) introduced a practical method for aminotransferase activity measurement. In 1955 Karmen et al. (3) developed an accurate and simple method that enabled the widespread use of this enzyme test in an ever-widening list of diseases (4). The majority of amino acids (except for lysine, threonine, proline and hydroxyproline) undergo transamination. However, 2 aminotransferases—aspartate aminotransferase [AST; EC 2.6.1.1; also known as serum glutamic oxaloacetic transaminase (SGOT or GOT)] and alanine aminotransferase [ALT; EC 2.6.1.2; also known as serum glutamic pyruvic transaminase (SGPT or GPT)]—are mostly metabolically active and abundant in cells. Both enzymes are routinely measured mostly to diagnose liver disease/injury, monitor therapy or assess disease course and prognosis of patients with liver disease. Evidence is growing that abnormal aminotransferase activity in circulation is associated with cardiovascular disease (CVD). The focus of this review is to summarize the evidence on the association between circulating ALT activity (thereafter as ALT) and CVD. The association between AST and CVD was not addressed.
ALT structure and functions
Human recombinant ALT produced in E. Coli is a non-glycosylated polypeptide chain with 495 amino acid residues and a molecular mass of 54,479 Dalton (5). The active enzyme is a dimer composed of two identical subunits with a molecular mass of 114,000 Dalton (5). Aminotransferases (including ALT) are pyridoxal-5‘-phosphate (PLP)-dependent enzymes. PLP is a vitamin B6 derivative that directly participates in the transamination reaction. So far 2 catalytically active isoforms of human ALT have been identified and called ALT1 and ALT2. There is also a third isoform (called ALT2_2) without enzymatic activity (6). The human ALT genes, GPT1 and GPT2, are located on chromosomes 8 (band 8q24.3.) and 16 (band 16q12.1.), respectively. The human GPT1 gene spans 2.7-kb and consists of 11 exons ranging in size from 79 to 243 base pairs and encodes a 495-amino acid residues polypeptide (7). The human GPT2 gene encodes a 3.9 kb mRNA, and has 12 exons spanning approximately 50 kb of the genome (8). The GPT2 gene encodes a polypeptide with 523 amino acid residues or a shorter polypeptide of 423 amino acid residues (ALT2_2) which is an alternative splice variant due to an alternative translational start codon usage (6).
Tissue expression of GPT1 and GPT2 differs considerably. In humans, GPT1 is expressed in liver, kidney, intestine, myocardium, skeletal muscle, colon, pancreas, spleen and lung (9). GPT2 gene is expressed in skeletal muscle, brain, heart and white adipose tissue (9). In other studies, a high GPT1 gene expression was detected in human liver, skeletal muscle and kidney, a low expression level was detected in myocardium and no expression was detected in pancreas. On the other hand, high GPT2 gene expression was detected in heart and skeletal muscle and no GPT2 expression was found in liver or kidney (10). Moreover, immunohistochemistry techniques have detected a strong ALT1 reactivity in hepatocytes, renal tubular and salivary gland epithelium whereas ALT2 reactivity was detected in adrenal gland cortex, neuronal cell body, cardiomyocytes, skeletal muscle and endocrine pancreas (10). Studies in isolated organelles have shown that ALT1 is located in cytosol and endoplasmatic reticulum of hepatocytes but not in mitochondria whereas ALT2 was located in mitochondria and endoplasmatic reticulum in skeletal muscle cells (6). Finally, both ALT1 and ALT2 contribute to ALT in plasma and immune-precipitation with ALT antibodies showed that ALT1 is mainly responsible for basal ALT in human plasma (10). Quantitatively, overall ALT in human liver, kidney, heart and muscle is 2,850, 1,200, 450 and 300 times higher than in serum (11). Although ALT in muscle is nearly 10-fold lower than in liver, considering the extent of muscular tissue (approximately 33 kg in healthy adults), skeletal muscle is the main reservoir of ALT in terms of quantity. Although, regulation of ALT expression remains poorly investigated, high protein intake, fasting, cortisol, glucagon, epinephrine and norepinephrine are reported to induce ALT expression in rat liver (12,13). There is also evidence that ALT2 expression is regulated by androgens via activation of promoter androgen response element(s) (14).
Aminotransferases have multiple metabolic functions. First, by catalyzing a freely reversible transfer of amino groups from amino acids to α-keto acids, aminotransferases create balanced proportions of amino acids according to the metabolic needs of the cells. Second, aminotransferases play a crucial role in the catabolism of amino acids by removing the amino group and amino acid synthesis from Krebs cycle intermediates. Aminotransferase reactions funnel amino groups from amino acids toward α-keto glutarate producing glutamate via ALT reaction. Glutamate is the only amino acid that undergoes large-scale deamination via enzyme glutamate dehydrogenase which effectively removes nitrogen from amino acids releasing ammonium and carbon skeleton of amino acids. The latter is further degraded for energy or used for other metabolic needs of the cells (i.e., gluconeogenesis). This increases the amount of Krebs cycle intermediates (called anaplerosis) and contributes to maintenance of oxidative capacity of the cells. Third, aminotransferases particularly ALT participate in the production and transport of ammonia (via glutamate dehydrogenase enzyme and amino acid glutamine). Fourth, depending on the metabolic needs and tissue, ALT reaction may provide alanine which serves as a vehicle for transporting pyruvate (after transformation to alanine by ALT in muscle) from contracting muscle to liver (transformed thereafter into pyruvate by ALT and glucose via glyconeogenesis in liver). Alanine is a major amino acid in blood in fasting conditions. Fifth, ALT reaction provides also glutamate which serves as a precursor for the synthesis of glutathione, a major antioxidant in cells. Moreover, glutamate is a neurotransmitter and a precursor of γ-amino butyric acid (GABA) in the brain (Figure 1).
ALT in circulation originates from multiple sources. Although the mechanism of ALT release from the cells remains unknown it is believed that it involves cellular leakage or cytoplasmic budding or blebbing into extracellular space and circulation. ALT1 accounts for most ALT in circulation and in clinically standard liver tests (10). However, ALT2 contributes to circulating ALT levels, typically in conditions like acute myocardial infarction or obesity (10). The current ALT assay measures combined ALT1 and ALT2 catalytic activity. The enzyme has a plasma half-life of 47±10 hours which is longer than that of AST (17±5 hours). ALT is cleared from circulation mostly via hepatic uptake (15). ALT shows a diurnal variation being up to 45% higher in the afternoon hours than in morning hours and a 10% to 30% day-to-day variability (16,17). ALT levels decrease with age for both men and women, independent of metabolic traits, alcohol use, and other markers of hepatic function, and consequently ALT may be considered a biomarker of aging (18).
Isolated congenital ALT deficiency is a very rare and in general a benign condition (19). The ALT silent gene called ALT0 gene was first reported by Olaisen in 1973 (20) and its frequency in the Caucasian population is estimated to be about 2.5 in 1,000 (21). In a case report of an ALT deficiency in a Japanese women with hepatitis C, low ALT was also observed in her 2 sons (22). Recent studies have shown that loss-of-function mutations in the ALT2 gene are associated with severe neurological alterations, developmental encephalopathy (23) or postnatal microcephaly, and spastic paraplegia with progressive features and intellectual and developmental disability (24). Acquired ALT deficiency is observed in the setting of vitamin B6 deficiency in patients with cirrhosis or chronic kidney failure on dialysis (25).
ALT and CVD
Prospective cohort studies have produced strikingly conflicting results with respect to the association between ALT and CVD or total (or CVD-related) mortality. Some studies have reported a positive association between ALT and CVD or mortality. Other studies have reported a negative, neutral or U-shaped relationship (Table 1).
Table 1
| Author (year) (reference) | Type of study | Number of participants (age) | Follow-up (years) | Alanine aminotransferase cutoff | Outcome/adjusted risk estimate | P value |
|---|---|---|---|---|---|---|
| Arndt et al. (1998) (26) | Cohort | 7,858 construction workers (25–64 years) | 5 | >22 U/L | Total mortality: RR =1.3 (0.9–1.9) | Not given |
| Vocational disability: RR =1.3 [1.0–1.7) | ||||||
| Kim et al. (2004) (27) | Prospective cohort | 94,533 men/47,522 women (35–59 years) | 8 | ≥100 (men) | Total mortality: RR =5.2 (4.2–6.4) | Not given |
*, reference range 8–41 U/L for women and 9–59 U/L for men. ALT, alanine amino transferase; CVD, cardiovascular disease; Diab-rel., diabetes related; HR, hazard ratio; ICH, Intracerebral hemorrhage; IHD, ischemic heart disease; LOI, loss of independence; MACE, major adverse cardiovascular events; NHANES III, third National Health and Nutrition Examination Study; NS, not significant; RR, relative risk; SD, standard deviation; ULN, upper limit of normal.
Elevated ALT and CVD and mortality
A number of studies have reported increased risk of CVD or mortality associated with higher ALT levels. Arndt et al. (26) investigated the association of liver enzymes with vocational disability and mortality in male construction workers in Southern Germany between 1986 and 1988. Elevated activity of gamma-glutamyl transferase (GGT), ALT and AST was observed in 32%, 22% and 12% of subjects, respectively. Self-reported alcohol consumption, diabetes and arterial hypertension were strongly associated with elevated levels of all three enzymes whereas body mass index (BMI) was strongly associated with GGT and AST elevation but not with ALT. Notably GGT and AST but not ALT were strongly associated with early retirement and all-cause mortality. Kim et al. (27) investigated the association of liver enzymes (ALT and AST) with all-cause and cause-specific (cancer, CVD and digestive tract diseases) mortality in a large cohort study in Korean population. ALT elevation was associated with higher risk of all-cause and CVD-related mortality in men with a dose-response relationship for values from <20 to ≥100 U/L. Results in women were inconsistent with respect to all-cause mortality whereas CVD mortality was not calculated due to paucity of events. A cohort study of community dwelling individuals in Japan found a significant ALT-by-BMI interaction. In individuals with BMI below the median value (22.7 kg/m2) there was a >8-fold increase in the risk of 10-year mortality compared with an insignificant 1.38-fold increase in individuals with BMI ≥ the median value (both adjusted risk estimates calculated for ALT ≥50 vs. <20 U/L). The increased risk associated with higher ALT in lean participants was explained by chronic liver disease associated with low BMI in the baseline survey (28). Of 6,823 adult residents of Olmsted County, Minnesota, who had a health care encounter at Mayo Clinic, Rochester in 1995, ALT higher than upper limit of normal (ULN) was found in 13% of them. Higher ALT was associated with higher standardized mortality ratio which was significant for ALT >2× ULN. Conversely, ALT within the normal range was associated with lower risk of death [standardized mortality ratio =0.61 (0.53–0.71); P<0.001]. Deaths were mostly due to hepatobiliary diseases (32). In a survey of 37,085 subjects undergoing health examinations at a health promotion center between 2000 and 2001 in Seoul, Korea, subjects with ALT in the 4th quartile had a 2.28-fold higher adjusted risk for CVD- or diabetes-related mortality over a median follow-up of 5 years (34).
Recent studies in Asian population provided further support for an association between higher ALT and mortality. Of 54,751 males in Taiwan undergoing health screening from 1996 to 2003 who were free of cancer at baseline, a random cohort of 3,961 males was selected and compared with 1864 males who died. After adjustment, higher ALT levels were associated with all-cause mortality and cancer mortality but not with CVD mortality. However, if ALT was entered into the model after log transformation, ALT was inversely associated with risk of mortality [hazard ratio (HR) =0.69, 85% confidence interval (CI) 0.53 to 0.91] (40). The association between ALT and intracerebral hemorrhage incidence and mortality in men and women was investigated in a large study from the East Asian Network for Stroke Prevention. After adjustment for age, blood pressure, diabetes, total cholesterol, smoking and alcohol intake, for each 10 U/L higher ALT, the intracerebral incidence and mortality increased by 4% (each) in men and 1% (insignificant) and 4% in women, respectively (41). In the largest, so far, study that included >16 million adult Koreans, Choi et al. (52) showed a significant association of ALT with total mortality, ischemic stroke and myocardial infarction after adjustment for age, sex, BMI, smoking, alcohol, exercise, diabetes, hypertension, and dyslipidemia. The risk for total mortality, ischemic stroke and myocardial infarction increased by 17%, 6% and 10%, respectively (4th vs. 1st ALT quartiles). ALT showed a U-shaped association with mortality.
Several studies have been neutral or have reported mixed results in terms of association between ALT and CVD or mortality. In the 10-year follow-up of the Hoorn study, age- and sex-adjusted HRs for all-cause mortality, incident CVD events and ischemic heart disease (IHD) events were 1.30 (0.92–1.83), 1.40 (1.09–1.81) and 2.04 (1.35–3.10), respectively, for 3rd vs. 1st ALT tertile. However, after adjustment for age, sex, alcohol-intake, smoking, physical activity, waist circumference, triglycerides, systolic blood pressure, fasting glucose and high-density lipoprotein (HDL)-cholesterol, the association with all-cause mortality and CVD events was attenuated, whereas the association with IHD remained significant (30). The Framingham Offspring Heart Study showed that the risk for the development of metabolic syndrome and diabetes was increased in subjects with higher ALT over 20 years of follow-up. There was an increased risk of CVD in age-sex adjusted models [HR =1.23 (1.12–1.34); P<0.001] which was attenuated after multivariable adjustment. There was no association between ALT and all-cause mortality (31). In the third US National Health and Nutrition Examination Survey (NHANES) that included 14,950 adult participants who were negative for markers of viral hepatitis B and C, elevated ALT was found in 13.5% of the participants. Subjects with ALT elevation were younger and more likely to be Mexican American, obese, diabetic, lighter smokers, and less physically active, and to have a central fat distribution, higher total cholesterol, diastolic blood pressure, serum transferrin saturation, more prevalent elevated C-reactive protein and lower HDL-cholesterol. Over a median of 8.8 years, cumulative all-cause and CVD mortality were 13.9% and 4.2%, respectively. After adjustment, elevated ALT was not associated with all-cause or CVD mortality but was associated with >8-fold higher adjusted risk for mortality due to liver disease (35). The Longitudinal Study of Aging found no strong evidence of an association between ALT and all-cause mortality after controlling for potential confounders. Additionally, the intra-pair analysis in which one twin had higher ALT found no strong evidence that higher ALT was associated with earlier death and the results were consistent in both monozygotic and dizygotic twins (33). A 2012 report from the NHANES study demonstrated that diabetes is a modifier of the association between ALT and mortality. Specifically, ALT was positively associated with mortality from diabetes among men and mortality from IHD related to diabetes and negatively with mortality unrelated to diabetes after adjustment for age, gender, education, race/ethnicity, smoking, and alcohol use (39). A retrospective analysis of data from the National Health Insurance Corporation in South Korea including >300,000 subjects receiving medical health check-ups from 2002 to 2008 showed a U-shaped relationship between ALT and risk of mortality in subjects ≥60 years of age (48). Sustainability of ALT elevation seems to be also important. In one study in men (n=68,431), only the group with sustained ALT elevation in 2 screening measurements [adjusted HR =2.29 (1.27–4.12)] but not the group with normalization [adjusted HR =1.35 (0.70–2.61)] showed higher risk of CVD mortality. Total mortality remained elevated in sustained [HR =1.41 (1.10–1.80)] and normalized [HR =1.38 (1.06–1.80)] elevation groups (55).
In aggregate, evidence linking elevated ALT levels with CVD or mortality is inconsistent. The association between elevated ALT and mortality appears to be stronger in studies in Asian populations compared to US or European populations. The association between ALT and CVD and mortality seems to be stronger in men than women. However this should be interpreted with caution due to the lower prevalence of CVD which renders some sex-based comparative analyses inconclusive due to lower rates of incident CVD (or CVD events) in women. Finally, elevated ALT seems to correlate with metabolic liver disease (primarily non-alcoholic fatty liver disease) and CVD risk factors. Consequently, several studies have reported an attenuation of the association between elevated ALT and incident CVD or mortality after adjustment for these factors. The association between elevated ALT and CVD risk factors is addressed later in this review.
Inverse association between ALT and CVD or mortality
Over the last two decades, evidence has accumulated that lower ALT levels are associated with increased risk of overall and CVD mortality. A 2006 prospective cohort study by Elinav et al. (29) showed a significantly lower survival in men with ALT below the median (13 U/L) versus men with ALT > median (54% vs. 65%) corresponding to a 2.42-fold higher adjusted risk for 12-year mortality. No such association was observed in women with ALT < or > median (11 U/L). The negative association between low ALT and mortality was explained by age-related comorbidities, hepatic aging and possibility that subjects high-normal ALT might have already died by the time the study had started. Moreover direct association between ALT and BMI and a high AST/ALT ratio implicated worse nutritional status and occult pyridoxal-5’-phosphate deficiency as putative contributors to increased mortality (29). In a cohort study by Hovinen et al. (36) age-specific (men: 31, 27, and 24 U/I for 75, 80, and 85–90 years, respectively; women: 29, 27, and 23 IU for 75, 80, and 85–90 years, respectively) high ALT was found in 45 women (17.4%) and 27 men (19.6%). Overall, 127 participants (32.0%) died, and low ALT was associated with lower survival in men (log rank P=0.002) and women (log rank P=0.03). In age-adjusted Cox model, logarithmic ALT was independently associated with mortality in men [HR =0.19 (0.06–0.62); P=0.006 for 1 unit higher log ALT] but not in women [HR =0.27 (0.07–1.08); P=0.06 for 1 unit higher log ALT]. Sex-specific adjusted HRs are shown in Table 1. In 1,673 community-dwelling men ≥70 years of age, participants with ALT below the median value had reduced survival up to 4.9 years of follow-up compared with participants with ALT > median. ALT was lower in older participants (24.9±18.0 U/L in the 70- to 74.9-year-old participants vs. 16.8±7.8 U/L in the participants >90 years; P<0.002). Of note, low ALT was associated with frailty [odds ratio (OR) =3.54 (2.45–5.11)] and the association between ALT and survival was attenuated once frailty and age were entered into the model (37). In a 2011 publication by Ford et al. (38) that included three independent populations (WOSCOPS and PROSPER trials that explicitly excluded subjects with clinically significant liver damage and Leiden 85-plus study of survivors to age 85 years) reported a consistent negative association between ALT and total mortality across all populations. In the WOSCOPS trial, ALT was inversely associated with non-CVD deaths (P=0.0061), cancer deaths (P=0.021), composite of IHD death or hospitalization (P=0.029) and CVD death or hospitalization (P=0.0003) and fatal and nonfatal stroke (P=0.034). In the PROSPER trial, ALT was inversely associated with IHD deaths (P=0.0037), non-CVD deaths (P=0.017), IHD death or nonfatal myocardial infarction (P=0.0042) and CVD death (P=0.040). In the Leiden 85-plus study, ALT was significantly associated with the risk for total mortality only (38). A recent report from the NHANES 1988–1994 survey showed that mortality risk was significantly higher in subjects with ALT in decile 1 [HR =1.42 (1.24–1.63)], decile 2 (HR =1.27 [1.06–1.53]) and decile 3 [HR =1.25 (1.04–1.50)] and non-significantly higher in decile 10 [HR =1.21 (0.91–1.61)] compared with subjects with ALT in deciles 4 to 9. It was hypothesized that low ALT was associated with higher mortality risk, possibly attributable to decreased appendicular lean mass assessed by dual-energy X-ray absorptiometry (56).
In the last 5 years additional evidence supporting a negative association between ALT and the risk of mortality has been gathered. Ramaty et al. (42) showed in a large cohort of adults (48±11 years of age) with ALT within normal range that ALT levels <17 U/L were associated with a significant 60% higher risk of total mortality over an 8.5-year follow-up. The association remained significant after adjustment for age, gender, glomerular filtration rate, albumin, arterial hypertension, diabetes mellitus and IHD. Another cohort study by Koehler et al. (43) showed a J-shaped relationship between ALT and the risk of mortality. Subjects with ALT <25th percentile (<12 U/L for women and <13 U/L for men) and those with ALT ≥95th percentile (33 U/L for women and 35 U/L for men) showed the highest risk of mortality. The association of ALT with CVD-related mortality was not significant. The Bezafibrate Infarction Prevention (BIP) registry showed an 11% increased adjusted risk for total mortality in subjects with ALT <17 U/L compared with subjects with ALT ≥17 U/L over a 22.8-year follow-up (47). The study identified older age, lower BMI, female sex as independent correlates of low ALT. A study by McCallum et al. (44) with 12,000 hypertensive subjects showed an independent inversely linear association between ALT and total mortality and mortality due to CVD and IHD. Kunutsor et al. (45) showed a significant 12% decrease in the adjusted risk for incident CVD or incident IHD and an insignificant 8% lower risk for stroke for each SD higher log ALT. An analysis of patients recruited in a recent randomized trial on statin efficacy showed that each higher SD unit of ALT was associated with 18% lower risk of incident CVD (defined as CVD death, stroke, myocardial infarction, hospitalization for unstable angina and arterial revascularization). The statin efficacy was not modified by baseline ALT (49). A recent study in Japanese elderly showed an association between low ALT (ALT values <10 U/L or 10–20 U/L) and the risk for death or loss of independence over a median of 5.75 years compared with ALT reference (20–30 U/L) values (53). Subjects with ALT values 30–40 or ≥40 U/L, did not show a higher risk for death or loss of independence [HR =1.29 (0.72–2.31) and HR =1.49 (0.68–3.25), respectively]. Our group investigated the association between ALT and mortality in 9,523 patients with angiography-confirmed IHD over a 3-year follow-up. The study showed a significant 43% higher risk of cardiac mortality, an insignificant 19% higher risk of total mortality and no association between ALT (for 1 unit decrement in log ALT scale) and the risk for stroke or myocardial infarction. Of note, ALT increased significantly but modestly the C-statistic of the multivariable model for prediction of cardiac mortality showing an improvement in risk prediction for mortality by this biomarker. The study raised the hypothesis that a low ALT level reflects CVD risk that is poorly mediated by traditional CVD risk factors (54). Two studies in adult subjects with type 2 diabetes showed an inverse relationship between ALT and the risk for total mortality (46,50). However, the findings were discordant with respect to the association between low ALT and the risk of CVD mortality. One study (51) showed an association between low ALT and the risk for incident CVD (defined as nonfatal myocardial infarction, stroke, coronary and other CVD death, coronary or carotid revascularization).
The association between ALT and the risk for CVD or mortality has been investigated in Mendelian randomization studies and meta-analyses. A Mendelian randomization study showed that genetically predicted ALT was associated with higher risk of diabetes [OR =2.99 (1.62–5.52) whereas the association with IHD/myocardial infarction was questionable [OR =0.74 (0.54–1.01)]. ALT and other liver enzymes tended to be inversely related to both low-density lipoprotein (LDL)- and HDL-cholesterol (57). Another recent Mendelian randomization study showed that ALT was negatively associated with IHD [OR =0.92 (0.87–0.97)] and triglycerides (coefficient beta =−0.08) but not with other CVD risk factors (58). A 2014 meta-meta-analysis of 12 prospective studies with 206,678 participants and 16 249 deaths, showed that the association between ALT and total or CVD-related mortality was modified by age. In older subjects (≥70 years of age), ALT was associated with lower risk of total [HR =0.91 (0.88–0.94)] and CVD-related [HR =0.91 (0.85–0.96)] mortality: in younger subjects (<70 years of age) the respective HRs were 1.06 (1.06–1.07) and 1.03 (1.02–1.05) calculated for 5 U/L of ALT increment (59). Another meta-analysis by Kunutsor et al. (60) showed no evidence of an association of ALT with CVD [relative risk (RR) =1.00 (0.99-1.02)]; however, ALT was inversely associated with IHD [RR =0.95 (0.90–1.00)] and positively associated with stroke [RR =1.01 (1.00–1.02)] with both of associations being of marginal significance. The heterogeneity across the studies was significant and potentially attributable to differences in baseline characteristics, cause-specific CVD outcome and length of follow-up and geographic location. A 2019 meta-analysis of 6 studies showed no independent association between ALT and CVD-related mortality [HR =0.89 (0.73–1.07); P=0.221] in the whole group of subjects and a significant inverse association between ALT and CVD-related mortality in subjects >55 years of age [HR =0.86 (0.75–0.99), P=0.001] (61).
In summary, evidence available strongly supports an independent, inverse and linear association between ALT and the risk for CVD or mortality. The inverse association was more frequently reported in studies that have included subjects with ALT within normal range. This might have minimized the risk associated with abnormal higher ALT level (mostly due to underlying liver disease) and its contribution to total mortality and pattern of relationship. There is evidence to suggest that the association between ALT and mortality may differ according to age, sex, diabetes, obesity and geographic location. There is no convincing evidence supporting an association between ALT and the risk for acute coronary events. Limited evidence suggests that low ALT may improve risk prediction for mortality by providing prognostic information poorly presented by traditional CVD risk factors. There appears to be a positive association between ALT and the risk of stroke, which is of marginal statistical significance and doubtful clinical meaning.
Mechanism of association of high and low ALT with CVD
Knowledge of the underlying mechanisms of the association between ALT and the risk of CVD or mortality is incomplete and remains hypothetical. Before addressing putative mechanisms of increased risk associated with high or low ALT, 3 aspects may be discussed. First, although ALT (an aminotransferases in general), catalyze reactions that are of fundamental metabolic importance, the association of ALT with CVD risk is hardly explainable with transaminase reaction per se. Second, there are no known specific metabolic functions of circulating aminotransferases. Third, there is no evidence to suggest that circulating aminotransferase activity correlates with enzyme activity in cytoplasm of the cells. Thus in order to explain the risk associated with ALT, attention should be focused on factors or conditions that are associated with high or low ALT levels. Putative mechanisms of the association of high and low ALT with CVD risk are separately discussed (Figure 2).
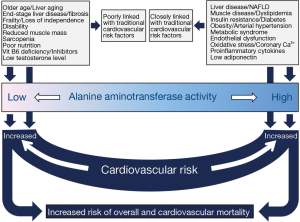
The association between elevated ALT and CVD risk is explainable by association of this condition with an array of CVD risk factors. Muscle diseases and injury lead to elevated aminotransferase levels and may mediate a poor prognosis. However, the most important mechanism explaining CVD risk associated with higher ALT values is clinical or subclinical underlying liver disease, particularly nonalcoholic fatty liver disease. ALT was shown to correlate with liver fat measured by magnetic resonance spectroscopy both in men and women (62,63). Nonalcoholic fatty liver disease is common and is associated with an array of metabolic disorders for which it is considered as a metabolic syndrome equivalent. ALT correlates with hepatic insulin resistance, insulin secretion, and glucagon level in healthy men and women (64). In adult subjects with no serologically diagnosable chronic liver diseases or excessive alcohol consumption, ALT was significantly associated with metabolic syndrome and all its components and the association remained significant after adjustment for insulin resistance (65). Elevated ALT was positively associated with atherogenic lipids such as apolipoprotein B, triglycerides and small dense LDL particles and high plasma glucose, CVD risk factors such as abdominal obesity and high blood pressure and negatively with atheroprotective lipoproteins such as, apolipoprotein A1 and HDL-cholesterol, potentially due to underlying nonalcoholic fatty liver disease (36,66-69). The association of ALT and plasminogen activator inhibitor-1 antigen, factor XIII B subunit, and factor XII indicating an increased thrombotic risk associated with elevated ALT has been reported (68). Associations of elevated ALT with the Framingham risk score (70,71), endothelial dysfunction (72), coronary calcification (73), presence (74) and severity (75) of coronary artery disease, C-reactive protein (36) and low adiponectin (76) have been reported. These and other studies clearly show that an elevated ALT is a correlate of CVD risk mostly due to underlying liver disease. Thus, it comes to no surprise that the association between ALT and the risk for CVD or mortality is vulnerable and is frequently attenuated after adjustment for CVD risk factors and liver disease. Moreover, liver disease related mortality may be a great contributor to total mortality in studies investigating the association between ALT and this outcome.
Although the association between low ALT and increased risk for CVD or mortality remains obscure, several explanations have been offered. Low ALT was shown to be associated with advanced age (37). Since ALT in the blood is primarily hepatic in origin, the association between low ALT and age was explained by age-related hepatic aging characterized by reduced liver size and blood flow and histological alterations presumably due to chronic oxidative stress (29,38). Moreover, hepatic diseases characterized by large-scale fibrosis substituting large parts of liver parenchyma may lead to low ALT due to reduced production and release of the enzyme, commonly found in association with other markers of reduced hepatic function such as low albumin and cholesterol. Low ALT is also associated with frailty (37) and loss of independence (53) and is explained by mechanisms similar to those explaining lower ALT with aging and occult diseases commonly coexisting with these syndromes, particularly in elderly. Low ALT has been associated with sarcopenia (56) a well-known risk factor for mortality. It has been recently reported that low ALT in subjects ≥65 years of age without chronic liver disease, malignancies or alcohol abuse is a marker of frailty, disability, and sarcopenia and an independent correlate of reduced survival (77). Malnutrition and pyridoxal-5’-phosphate deficiency have also been implicated as a possible factor of poor survival associated with low ALT levels (29). Since ALT correlates with BMI, lower BMI values in subjects with low ALT have been suggested to reflect worse nutritional status (29). Pyridoxal-5’-phosphate deficiency, either isolated or in the setting of malnutrition may lead to low ALT and poor subsequent outcomes (77). If ALT in circulation parallels ALT in cells, then lower rates of transamination may lead to metabolic consequences such as reduced rates of glyconeogenesis and reduced oxidative capacity of the cells. Low testosterone level may lead to low ALT levels, either via androgen participation in ALT expression (14) or as part of frailty syndrome (78) in men. Since low testosterone levels is common in elderly and correlates with markers of atherosclerotic heart disease in diabetic patients (79) or reduced survival in patients with IHD (80), it may help to explain increased CVD risk associated with low ALT. However this hypothesis requires testing. One characteristic of almost all factors postulated to explain the association between low ALT levels and increased risk of mortality or CVD is that they are poorly (if at all) linked with traditional risk factors. Information on these factors is not readily available and consequently most studies did not adjust for them. They remain unaccounted for and are qualified as residual confounders. Thus, under-adjustment for these factors appears to increase the likelihood of finding an inverse association between low ALT and CVD or mortality mostly in studies that have excluded subjects with elevated ALT levels.
Concluding remarks
Evidence linking ALT with CVD or mortality remains incomplete and controversial. In various studies, positive, negative, neutral and J- or U-shaped relationships between ALT and CVD or total (or CVD-related) mortality have been reported. Although the reasons for such a high magnitude of controversy across the studies remain poorly understood, some putative explanations exist. Thus, in studies that have included subjects without restrictions in terms of ALT level, a positive, neutral or U-shaped relationship between ALT and CVD outcomes appears to be more likely. This sounds reasonable considering that this approach includes both categories of CVD risk, i.e., CVD risk associated with high ALT levels (underlying a positive association between ALT and CVD outcomes) and the risk associated with low ALT levels (underlying a negative association between ALT and CVD outcomes). Conversely in studies that have included only subjects with ALT within the reference range, i.e., excluding those with abnormal (high) ALT levels, an inverse association between ALT and CVD outcomes appears to be more likely. In addition evidence available suggests that the association between ALT and CVD or total (or CVD-related) mortality may differ according to age (more likely to be positive in younger and inverse in older subjects), sex (stronger in men), diabetes (more likely to be positive in diabetic subjects and inverse in nondiabetic subjects), obesity (stronger association in lean subjects) and geographic location (stronger association in Asian population). In case of a non-linear (J- or U-shaped) relationship between ALT and outcomes of interest, the use of appropriate statistical test (i.e., restricted cubic spline regression or other) is needed otherwise the true association may remain undetected. The related mechanisms can be grouped into two categories (Figure 2). The first category includes risk factors that tend to cluster in subjects with high ALT levels and most of them are directly or indirectly related to liver inflammation or nonalcoholic fatty liver disease. They are closely linked with CVD risk factors and this may explain the positive association between high ALT and increased risk of CVD or mortality and susceptibility of this association to adjustment for traditional CVD risk factors. The second category of risk includes risk factors which tend to cluster in subjects with low ALT level (advanced age, hepatic aging, frailty, sarcopenia, malnutrition and occult diseases associated with these conditions). These risk factors are poorly linked with traditional CVD risk factors and this may explain the inverse association between ALT and CVD or mortality. Commonly these risk factors remain unaccounted for. The current level of evidence does not allow a firm recommendation of ALT measurement for CVD risk prediction. However, aminotransferases are commonly measured for health assessment and subjects with normal-low or high ALT activity may need screening for CVD risk factors. As recently suggested, low enzyme activities (including ALT) have clinical meaning and should be reported and analyzed (81). Finally, future dedicated and well-conducted epidemiological and clinical studies are needed to better clarify the association between ALT and the risk for CVD or mortality.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.08.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Braunstein AE, Kritzmann MG. Decomposition and synthesis of amino acids by conversion of amines; studies on muscle tissue. Enzymologia 1937;2:129-46.
- Cammarata PS, Cohen PP. Spectrophotometric measurement of transamination reactions. J Biol Chem 1951;193:45-52. [PubMed]
- Karmen A, Wroblewski F, Ladue JS. Transaminase activity in human blood. J Clin Invest 1955;34:126-31. [Crossref] [PubMed]
- Agress CM. Evaluation of the transaminase test. Am J Cardiol 1959;3:74-93. [Crossref] [PubMed]
- Ishiguro M, Takio K, Suzuki M, et al. Complete amino acid sequence of human liver cytosolic alanine aminotransferase (GPT) determined by a combination of conventional and mass spectral methods. Biochemistry 1991;30:10451-7. [Crossref] [PubMed]
- Glinghammar B, Rafter I, Lindstrom AK, et al. Detection of the mitochondrial and catalytically active alanine aminotransferase in human tissues and plasma. Int J Mol Med 2009;23:621-31. [Crossref] [PubMed]
- Sohocki MM, Sullivan LS, Harrison WR, et al. Human glutamate pyruvate transaminase (GPT): localization to 8q24.3, cDNA and genomic sequences, and polymorphic sites. Genomics 1997;40:247-52. [Crossref] [PubMed]
- Yang RZ, Blaileanu G, Hansen BC, et al. cDNA cloning, genomic structure, chromosomal mapping, and functional expression of a novel human alanine aminotransferase. Genomics 2002;79:445-50. [Crossref] [PubMed]
- Yang RZ, Park S, Reagan WJ, et al. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 2009;49:598-607. [Crossref] [PubMed]
- Lindblom P, Rafter I, Copley C, et al. Isoforms of alanine aminotransferases in human tissues and serum--differential tissue expression using novel antibodies. Arch Biochem Biophys 2007;466:66-77. [Crossref] [PubMed]
- Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev 2013;34:117-30. [PubMed]
- Rosen F, Roberts NR, Nichol CA. Glucocorticosteroids and transaminase activity. I. Increased activity of glutamic pyruvic transaminase in four conditions associated with gluconeogenesis. J Biol Chem 1959;234:476-80. [PubMed]
- Begum NA, Datta AG. Effect of adrenergic agonists and antagonists on alanine amino transferase, fructose-1:6-bisphosphatase and glucose production in hepatocytes. Mol Cell Biochem 1992;113:93-103. [Crossref] [PubMed]
- Coss CC, Bauler M, Narayanan R, et al. Alanine aminotransferase regulation by androgens in non-hepatic tissues. Pharm Res 2012;29:1046-56. [Crossref] [PubMed]
- Price C, Alberti K. Biochemical assessment of liver function. In: Wright R. editor. Liver and biliary diseases—pathophysiology, diagnosis, management. London: WB Saunders, 1979:381-416.
- Córdoba J, O'Riordan K, Dupuis J, et al. Diurnal variation of serum alanine transaminase activity in chronic liver disease. Hepatology 1998;28:1724-5. [Crossref] [PubMed]
- Fraser CG. Biological variation in clinical chemistry: an update: collated data, 1988-1991. Arch Pathol Lab Med 1992;116:916-923. [PubMed]
- Dong MH, Bettencourt R, Brenner DA, et al. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clin Gastroenterol Hepatol 2012;10:285-290.e1. [Crossref] [PubMed]
- Kömpf J, Ritter H. Polymorphism of alanine aminotransferase (E.C.2.7.6.1): common and rare alleles. Hum Genet 1979;51:287-92. [Crossref] [PubMed]
- Olaisen B. Atypical segregation of erythrocyte glutamic-pyruvic transaminase in a Norwegian family. Evidence of a silent allele. Hum Hered 1973;23:595-602. [Crossref] [PubMed]
- Sparkes MC, Crist M, Sparkes RS. Glutamate pyruvate transaminase null allele in seven new families. Hum Genet 1983;65:147-8. [Crossref] [PubMed]
- Uno S, Kaito M, Kobayashi Y, et al. Case report: Alanine aminotransferase deficiency detected in a patient with chronic hepatitis C. J Gastroenterol Hepatol 1998;13:480-2. [Crossref] [PubMed]
- Celis K, Shuldiner S, Haverfield EV, et al. Loss of function mutation in glutamic pyruvate transaminase 2 (GPT2) causes developmental encephalopathy. J Inherit Metab Dis 2015;38:941-8. [Crossref] [PubMed]
- Ouyang Q, Nakayama T, Baytas O, et al. Mutations in mitochondrial enzyme GPT2 cause metabolic dysfunction and neurological disease with developmental and progressive features. Proc Natl Acad Sci U S A 2016;113:E5598-607. [Crossref] [PubMed]
- Ono K, Ono T, Matsumata T. The pathogenesis of decreased aspartate aminotransferase and alanine aminotransferase activity in the plasma of hemodialysis patients: the role of vitamin B6 deficiency. Clin Nephrol 1995;43:405-8. [PubMed]
- Arndt V, Brenner H, Rothenbacher D, et al. Elevated liver enzyme activity in construction workers: prevalence and impact on early retirement and all-cause mortality. Int Arch Occup Environ Health 1998;71:405-12. [Crossref] [PubMed]
- Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004;328:983. [Crossref] [PubMed]
- Nakamura K, Okamura T, Kanda H, et al. The value of combining serum alanine aminotransferase levels and body mass index to predict mortality and medical costs: a 10-year follow-up study of National Health Insurance in Shiga, Japan. J Epidemiol 2006;16:15-20. [Crossref] [PubMed]
- Elinav E, Ackerman Z, Maaravi Y, et al. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc 2006;54:1719-24. [Crossref] [PubMed]
- Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis 2007;191:391-6. [Crossref] [PubMed]
- Goessling W, Massaro JM, Vasan RS, et al. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008;135:1935-44, 1944.e1.
- Lee TH, Kim WR, Benson JT, et al. Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008;47:880-7. [Crossref] [PubMed]
- Fraser A, Thinggaard M, Christensen K, et al. Alanine aminotransferase, gamma-glutamyltransferase (GGT) and all-cause mortality: results from a population-based Danish twins study alanine aminotransferase, GGT and mortality in elderly twins. Liver Int 2009;29:1494-9. [Crossref] [PubMed]
- Yun KE, Shin CY, Yoon YS, et al. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis 2009;205:533-7. [Crossref] [PubMed]
- Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009;136:477-85.e11. [Crossref] [PubMed]
- Hovinen SM, Pitkala KH, Tilvis RS, et al. Alanine aminotransferase activity and mortality in older people. J Am Geriatr Soc 2010;58:1399-401. [Crossref] [PubMed]
- Le Couteur DG, Blyth FM, Creasey HM, et al. The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci 2010;65:712-7. [Crossref] [PubMed]
- Ford I, Mooijaart SP, Lloyd S, et al. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. Int J Epidemiol 2011;40:1530-8. [Crossref] [PubMed]
- Schooling CM, Kelvin EA, Jones HE. Alanine transaminase has opposite associations with death from diabetes and ischemic heart disease in NHANES III. Ann Epidemiol 2012;22:789-98. [Crossref] [PubMed]
- Hernaez R, Yeh HC, Lazo M, et al. Elevated ALT and GGT predict all-cause mortality and hepatocellular carcinoma in Taiwanese male: a case-cohort study. Hepatol Int 2013;7:1040-9. [Crossref] [PubMed]
- Kim HC, Oh SM, Pan WH, et al. Association between alanine aminotransferase and intracerebral hemorrhage in East Asian populations. Neuroepidemiology 2013;41:131-8. [Crossref] [PubMed]
- Ramaty E, Maor E, Peltz-Sinvani N, et al. Low ALT blood levels predict long-term all-cause mortality among adults. A historical prospective cohort study. Eur J Intern Med 2014;25:919-21. [Crossref] [PubMed]
- Koehler EM, Sanna D, Hansen BE, et al. Serum liver enzymes are associated with all-cause mortality in an elderly population. Liver Int 2014;34:296-304. [Crossref] [PubMed]
- McCallum L, Panniyammakal J, Hastie CE, et al. Longitudinal Blood Pressure Control, Long-Term Mortality, and Predictive Utility of Serum Liver Enzymes and Bilirubin in Hypertensive Patients. Hypertension 2015;66:37-43. [Crossref] [PubMed]
- Kunutsor SK, Bakker SJ, Kootstra-Ros JE, et al. Inverse linear associations between liver aminotransferases and incident cardiovascular disease risk: The PREVEND study. Atherosclerosis 2015;243:138-47. [Crossref] [PubMed]
- Deetman PE, Alkhalaf A, Landman GW, et al. Alanine aminotransferase and mortality in patients with type 2 diabetes (ZODIAC-38). Eur J Clin Invest 2015;45:807-14. [Crossref] [PubMed]
- Peltz-Sinvani N, Klempfner R, Ramaty E, et al. Low ALT Levels Independently Associated with 22-Year All-Cause Mortality Among Coronary Heart Disease Patients. J Gen Intern Med 2016;31:209-14. [Crossref] [PubMed]
- Oh CM, Won YJ, Cho H, et al. Alanine aminotransferase and gamma-glutamyl transferase have different dose-response relationships with risk of mortality by age. Liver Int 2016;36:126-35. [Crossref] [PubMed]
- Harada PH, Cook NR, Cohen DE, et al. Relation of Alanine Aminotransferase Levels to Cardiovascular Events and Statin Efficacy. Am J Cardiol 2016;118:49-55. [Crossref] [PubMed]
- Williams KH, Sullivan DR, Nicholson GC, et al. Opposite associations between alanine aminotransferase and gamma-glutamyl transferase levels and all-cause mortality in type 2 diabetes: Analysis of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Metabolism 2016;65:783-93. [Crossref] [PubMed]
- Williams KH, Sullivan DR, Veillard AS, et al. Low alanine aminotransferase levels and higher number of cardiovascular events in people with Type 2 diabetes: analysis of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabet Med 2016;33:356-64. [Crossref] [PubMed]
- Choi KM, Han K, Park S, et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: A nationwide population-based cohort study. Sci Rep 2018;8:3764. [Crossref] [PubMed]
- Yamazaki H, Kamitani T, Matsui T, et al. Association of low alanine aminotransferase with loss of independence or death: A 5-year population-based cohort study. J Gastroenterol Hepatol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Ndrepepa G, Holdenrieder S, Colleran R, et al. Inverse association of alanine aminotransferase within normal range with prognosis in patients with coronary artery disease. Clin Chim Acta 2019;496:55-61. [Crossref] [PubMed]
- Lee H, Shin DW, Lee TH, et al. Association Between Change in Serum Aminotransferase and Mortality: A Nationwide Cohort Study in Korea. Medicine (Baltimore) 2016;95:e3158 [Crossref] [PubMed]
- Ruhl CE, Everhart JE. The association of low serum alanine aminotransferase activity with mortality in the US population. Am J Epidemiol 2013;178:1702-11. [Crossref] [PubMed]
- Liu J, Au Yeung SL, Lin SL, et al. Liver Enzymes and Risk of Ischemic Heart Disease and Type 2 Diabetes Mellitus: A Mendelian Randomization Study. Sci Rep 2016;6:38813. [Crossref] [PubMed]
- Xu L, Jiang CQ, Lam TH, et al. Mendelian randomization estimates of alanine aminotransferase with cardiovascular disease: Guangzhou Biobank Cohort study. Hum Mol Genet 2017;26:430-7. [PubMed]
- Liu Z, Ning H, Que S, et al. Complex association between alanine aminotransferase activity and mortality in general population: a systematic review and meta-analysis of prospective studies. PLoS One 2014;9:e91410 [Crossref] [PubMed]
- Kunutsor SK, Apekey TA, Khan H. Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis 2014;236:7-17. [Crossref] [PubMed]
- Rahmani J, Miri A, Namjoo I, et al. Elevated liver enzymes and cardiovascular mortality: a systematic review and dose-response meta-analysis of more than one million participants. Eur J Gastroenterol Hepatol 2019;31:555-562. [Crossref] [PubMed]
- Kotronen A, Yki-Jarvinen H, Sevastianova K, et al. Comparison of the relative contributions of intra-abdominal and liver fat to components of the metabolic syndrome. Obesity (Silver Spring) 2011;19:23-8. [Crossref] [PubMed]
- Westerbacka J, Corner A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia 2004;47:1360-9. [Crossref] [PubMed]
- Bonnet F, Ducluzeau PH, Gastaldelli A, et al. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011;60:1660-7. [Crossref] [PubMed]
- Olynyk JK, Knuiman MW, Divitini ML, et al. Serum alanine aminotransferase, metabolic syndrome, and cardiovascular disease in an Australian population. Am J Gastroenterol 2009;104:1715-22. [Crossref] [PubMed]
- Siddiqui MS, Sterling RK, Luketic VA, et al. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology 2013;145:1271-9.e1-3.
- Lorenzo C, Hanley AJ, Rewers MJ, et al. The association of alanine aminotransferase within the normal and mildly elevated range with lipoproteins and apolipoproteins: the Insulin Resistance Atherosclerosis Study. Diabetologia 2013;56:746-57. [Crossref] [PubMed]
- Kain K, Carter AM, Grant PJ, et al. Alanine aminotransferase is associated with atherothrombotic risk factors in a British South Asian population. J Thromb Haemost 2008;6:737-41. [Crossref] [PubMed]
- He KP, Zhao C, Qiang Y, et al. Impact of elevated aspartate and alanine aminotransferase on metabolic syndrome and its components among adult people living in Ningxia, China. Chronic Dis Transl Med 2015;1:124-32. [Crossref] [PubMed]
- Ioannou GN, Weiss NS, Boyko EJ, et al. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006;43:1145-51. [Crossref] [PubMed]
- Kim K, Kim DS, Kim KN. Serum Alanine Aminotransferase Level as a Risk Factor for Coronary Heart Disease Prediction in Koreans: Analysis of the Korea National Health and Nutrition Examination Survey (V-1, 2010 and V-2, 2011). Korean J Fam Med 2019;40:124-8. [Crossref] [PubMed]
- Schindhelm RK, Diamant M, Bakker SJ, et al. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus. Eur J Clin Invest 2005;35:369-74. [Crossref] [PubMed]
- Jung DH, Lee YJ, Ahn HY, et al. Relationship of hepatic steatosis and alanine aminotransferase with coronary calcification. Clin Chem Lab Med 2010;48:1829-34. [Crossref] [PubMed]
- Shen J, Zhang J, Wen J, et al. Correlation of serum alanine aminotransferase and aspartate aminotransferase with coronary heart disease. Int J Clin Exp Med 2015;8:4399-404. [PubMed]
- Masoudkabir F, Karbalai S, Vasheghani-Farahani A, et al. The association of liver transaminase activity with presence and severity of premature coronary artery disease. Angiology 2011;62:614-9. [Crossref] [PubMed]
- Saito T, Nishise Y, Makino N, et al. Impact of metabolic syndrome on elevated serum alanine aminotransferase levels in the Japanese population. Metabolism 2009;58:1067-75. [Crossref] [PubMed]
- Vespasiani-Gentilucci U, De Vincentis A, Ferrucci L, et al. Low Alanine Aminotransferase Levels in the Elderly Population: Frailty, Disability, Sarcopenia, and Reduced Survival. J Gerontol A Biol Sci Med Sci 2018;73:925-30. [Crossref] [PubMed]
- Hyde Z, Flicker L, Almeida OP, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab 2010;95:3165-72. [Crossref] [PubMed]
- Farias JM, Tinetti M, Khoury M, et al. Low testosterone concentration and atherosclerotic disease markers in male patients with type 2 diabetes. J Clin Endocrinol Metab 2014;99:4698-703. [Crossref] [PubMed]
- Malkin CJ, Pugh PJ, Morris PD, et al. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 2010;96:1821-5. [Crossref] [PubMed]
- Delanghe JR, De Buyzere ML. Also low enzyme activities have a clinical meaning! Clin Chim Acta 2019;496:142. [Crossref] [PubMed]
Cite this article as: Ndrepepa G, Kastrati A. Alanine aminotransferase—a marker of cardiovascular risk at high and low activity levels. J Lab Precis Med 2019;4:29.



