
Tumour-induced hypoglycaemia: a narrative review
Introduction
Hypoglycaemia, defined by a plasma glucose level <3.0 mmol/L (54 mg/dL), results from failure to maintain normal plasma glucose levels through complex homeostatic mechanisms. Hypoglycaemia is a relatively common clinical scenario, particularly in patients with diabetes mellitus (DM), treated with insulin therapies or sulphonylurea drugs. Hypoglycaemia presents clinically with symptoms in three main categories: (I) autonomic sympatho-adrenal activation (sweating, tremor and palpitations); (II) neuroglycopenia (impaired mental concentration, aggressive behaviour, ataxia, blurred vision, impaired consciousness, impaired vision, speech and memory, paralysis, seizures and loss of consciousness), and; (III) malaise (nausea and headache) (1-3). Symptoms of hypoglycaemia vary according to age, ability to mount an effective sympatho-adrenal response and use of certain concurrent medications such as beta-blockers that effectively dampen or prevent any sympatho-adrenal response to hypoglycaemia (1).
The human brain relies upon a continuous supply of glucose, as an obligate fuel source (1). Therefore, the brain has an inherent vulnerability to any acute interruption to the supply of glucose, such as occurs during hypoglycaemia. Accordingly, complex evolutionary counter-regulatory mechanisms exist (that oppose the regulatory effect of insulin), to mitigate the potentially harmful and life-threatening consequences of hypoglycaemia (1). These include an increase in the endogenous production of glucose through glycogenolysis and gluconeogenesis, through a reduction in endogenous insulin release from the pancreatic beta cells, enhanced glucagon release from the pancreatic alpha cells, and finally release of adrenaline from the adrenal medulla that also promotes further release of hepatic glucose (1). To complement these important counter-regulatory physiological changes in response to acute hypoglycaemia, there is also a behavioural response, mediated through appetitive changes, to promote the increased consumption of food (1).
Although less common, hypoglycaemia that occurs outside of the context of DM and DM-related therapies (insulin and sulphonylureas), represents an important clinical scenario. Of the many potential causes of hypoglycaemia, a relatively uncommon, but nonetheless important group includes tumour-induced hypoglycaemia (TIH). TIH associates with multiple unique clinical challenges that relate primarily to the accurate and timely diagnosis and effective, individually tailored management. A prerequisite of the former is clinical suspicion of hypoglycaemia. Unfortunately, recurrent hypoglycaemia can be easily missed for a variety of reasons, including effective counter-regulatory responses (e.g., eating-behaviours), lack of a detailed and accurate history, a perceived lack of relevance and awareness amongst patients and healthcare professionals respectively, inadequate biochemical work-up, concurrent use of therapies such as beta-blockers and idiosyncratic and unusual presentations of hypoglycaemia, particularly in older patients. For these reasons, it seems likely that there may be a missed diagnosis of TIH in at least a proportion of patients. However, even when a clinical suspicion of recurrent hypoglycaemia prompts timely referral, the diagnostic work-up of TIH poses its own challenges, and essentially relies upon the occurrence of hypoglycaemia (either spontaneous or induced following a prolonged fast). There are specific challenges with effective management also, including the need for a multi-disciplinary approach.
In this concise narrative review, we provide an overview of the underlying pathogenesis and clinical features of TIH. We explore the diagnostic work-up for TIH, and effective management strategies. We also provide suggestions for how to address some of the common challenges encountered clinically in the accurate and timely diagnosis and effective management of TIH. We present the following article in accordance with the narrative review reporting checklist (available at https://dx.doi.org/10.21037/jlpm-20-110).
Methods
We performed a narrative literature review using PubMed and search-term “Tumour-Induced Hypoglycaemia”, with articles written in English.
Pathogenesis and clinical features of TIH
TIH is an umbrella term that incorporates any episode of hypoglycaemia that originates from an underlying tumour. Broadly, the two main categories of TIH include insulinoma and non-islet cell tumours (NICTs), the pathogenesis of each differing fundamentally. Accordingly, each group of tumours has a characteristic biochemical profile, and demands a specific and precise management strategy. It is hard to overstate the importance of obtaining an accurate and timely diagnosis of TIH, including the particular subtype, as this determines the formulation of an appropriate, individualised and effective management strategy. Misdiagnosis of TIH could have serious ramifications and result in inadequate and inappropriate treatment, with associated deleterious outcomes for the patient. In this section, we provide an overview of the pathogenesis and clinical features of each main category of TIH, insulinoma and NICTs, summarized in Table 1.

Full table
Insulinoma
Insulinomas are tumours of the pancreatic beta cells, first identified >90 years ago in a patient with co-occurrence of functional hyperinsulinaemia and an islet cell tumour (4). Insulinomas are usually small, sporadic and well circumscribed intra-pancreatic tumours, although can be distributed throughout the pancreas (5,6). Insulinomas are the commonest TIH-related cause of hypoglycaemia, the vast majority (between 90% and 95%) being benign (5), and malignancy occurring in only a small minority (5.8%) (6). Insulinomas are relatively rare with an incidence of 4 cases per million per year, based on a 60-year observation period in a US-based epidemiological study (6). Despite their rarity though, insulinomas are the commonest functioning pancreatic neuroendocrine tumours (NETs), accounting for 20–30% of such cases (6). In rare cases (between 4% and 6%), insulinomas associate with multiple endocrine neoplasia type 1 (MEN1) (5). Insulinomas occur frequently in the fifth decade, with a slight preponderance in women (5,6) and characteristically over-produce insulin, resulting in hyperinsulinaemia. Hypoglycaemia ensues, which results in stimulation of counter-regulatory responses. Importantly however, due to the autonomous over-production of insulin from the insulinoma-related tumour cells, there is a lack of appropriate suppression of insulin release, with ongoing propensity for recurrent hypoglycaemia.
The clinical features of insulinoma include “Whipple’s triad”: (I) onset of hypoglycaemic symptoms after fasting or heavy exercise; (II) biochemical confirmation of fasting hypoglycaemia, and; (III) relief of hypoglycaemic symptoms following restoration of normal blood glucose levels (7). The clinical features of insulinoma-related hypoglycaemia include symptoms typical of neuroglycopenia (8-10), and sympatho-adrenal activation (8-10). Classically, precipitation of symptoms of insulinoma-related hypoglycaemia occurs following fasting or exercise. However, based on data from a longitudinal study, a minority (6%) of cases of insulinoma present with just postprandial symptoms, and 21% of cases present with both fasting and postprandial symptoms (11). Of note, there is often a delayed diagnosis of insulinoma. Data from one study showed a median time to diagnosis of insulinoma of 24 months, and frequent confusion with seizure disorder and other neuropsychiatric disorders (5,12). Importantly, patients with insulinoma often experience persistent hunger, as an appropriate counter-regulatory behavioural response to recurrent hypoglycaemia. Changes in appetite can promote hyperphagia (including frequent small meals to lessen and prevent symptoms of hypoglycaemia), with resultant weight-gain and obesity as common clinical features of insulinoma (12).
NICTs
Recurrent hypoglycaemia from NICT origin is a paraneoplastic syndrome. NICTs mainly comprise tumours of mesenchymal origin such as sarcomas, fibromas, fibro sarcomas, solitary fibrous tumours, leiomyosarcomas, haemangiopericytomas and renal cell carcinomas (13,14). NICT-related hypoglycaemia usually stems from large and/or metastatic tumours, but can occur at any time during the natural disease course (13). Gastrointestinal stromal tumours (GIST) are the commonest mesenchymal tumours of the gastrointestinal tract, mainly affecting the stomach, and often asymptomatic until they cause symptoms related to mass effect such as gastrointestinal bleeding, perforation or obstruction (13). GIST can present with NICT-related recurrent hypoglycaemia prior to any other mass effect-related symptoms. NICTs differ fundamentally from insulinomas in their secretion profiles. Rather than over-production of insulin, NICTs produce and secrete excessive amounts of (incompletely processed) insulin-like growth factor 2 (IGF2). This incompletely processed and high-weight form of IGF2 is also referred to as “big”-IGF2. Overall, high serum levels of big-IGF2 cause hypoglycaemia through cross-reactivity with the insulin receptor, mediated by inhibited hepatic glycogenolysis, gluconeogenesis and glucose release, and continued glucose utilization by skeletal muscle (13). Although counter-regulatory mechanisms ensue, including suppression in the release of insulin from the pancreatic beta cells, autonomous production of big-IGF2 persists, and underlies chronic, persistent and recurrent hypoglycaemic episodes.
Each NICT is capable of production and secretion of large quantities of big-IGF2, with hepatocellular and gastric carcinomas the most common causes of NICT-related hypoglycaemia (15-17). Overall, recurrent hypoglycaemia predated the diagnosis of NICTs in 48% of cases in one multicentre study (17). However, the overall occurrence of NICT-related recurrent hypoglycaemia is a rarity; its incidence of 1 case per million population per year being around 25% that of insulinomas (14,18). The clinical features of NICT-related hypoglycaemia are similar to those of insulinoma described above. Classically, recurrent and persistent hypoglycaemia manifests with both neuroglycopenic features, sympatho-adrenal stimulation and the occurrence of Whipple’s triad (3). Although the clinical features of NICT-related TIH are usually restricted to the tumour itself and its associated recurrent hypoglycaemia, rarely other clinical features can also ensue. In one case report of a NICT resulting from a pelvic clear cell sarcoma, manifesting with hypoglycaemia and elevated serum levels of big-IGF2, the patient also presented with acromegaloid skin changes (soft tissue facial swelling, skin tags and nuchal hyperpigmentation), that resolved following resection of the underlying tumour and normalised serum big-IGF2 levels (19). This case highlights the potential for cross-reactivity of big-IGF2 with the insulin-like growth factor 1 (IGF1) receptor, with associated acromegaloid features.
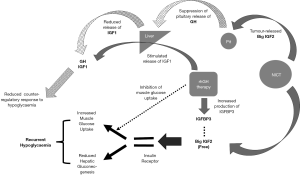
The pathogenesis of hypoglycaemia from NICTs implicates a central role for big-IGF2. In this context, it is also necessary to briefly discuss IGF1, the counterpart of IGF2; a detailed discussion of the physiology and regulation of IGF1 and IGF2 is beyond the scope of the present review, and has been previously reviewed elsewhere (20,21). In brief, both IGF1 and IGF2 are structurally similar to insulin and secreted primarily from the liver in normal physiology (20,22). Growth hormone (GH) stimulates the hepatic production of IGF1, whilst pituitary production of GH is itself regulated by serum levels of IGF1. In this way, GH and IGF1 form essential elements of the somatotropic axis. Conversely, IGF2 is independent of any influence from GH, and does not form any part of the somatotropic axis (20). Serum IGF1 and IGF2 act on the IGF1 and IGF2 receptors, respectively, in addition to cross-reactivity with the insulin receptor (23). Estimations show that the effect of IGFs on lowering plasma glucose levels (through their effects on the insulin receptor) is 10-fold less than that of a similar serum concentration of insulin. However, although the serum concentration of IGFs is around 1,000-fold greater than that of insulin (24,25), the majority of IGFs (90%) within the circulation are bound to IGF-binding proteins (IGFBPs), with IGFBP3 binding to over 95% of circulating IGFs, thereby blocking their plasma glucose-lowering potential (26,27). Importantly though, as aforementioned, the big-IGF2 produced from NICTs is a larger, immature peptide that stems from abnormal post-translational processing, due to insufficient enzyme-related modification (20,21). Big-IGF2 has a lesser affinity for binding to IGFBPs. Accordingly, the free component of big-IGF2 within the serum is 20-fold greater than that of IGF2. This results in a greater glucose-lowering potential of big-IGF2 (from cross-reactivity of the free-component of big-IGF2 with the insulin receptor), compared to that of IGF2 (much of which is bound to IGFBPs and therefore comparatively less cross-reactive with the insulin receptor) (28). The increased action of free (unbound) serum big-IGF2 on peripheral insulin receptors results in increased glucose uptake into cells, and consequent hypoglycaemia. Increased serum levels of free big-IGF2 in patients with NICTs can also disrupt the production of GH from the pituitary through cross-reactivity with IGF1 receptors and therefore enhanced negative feedback effects, with consequent diminishment of downstream hepatic IGF1 release, thereby disrupting one of the counter-regulatory responses to hypoglycaemia, and further contributing towards persistent and recurrent hypoglycaemic episodes (21,28). We provide an overview of the pathogenesis of hypoglycaemia in TIH from NICT in Figure 1.
Diagnosis of TIH
Having considered the pathogenesis and clinical features of insulinoma and NICT-related hypoglycaemia, it is important to consider the diagnostic challenges of these two conditions. As outlined, an accurate and timely diagnosis of TIH is essential for the implementation of an appropriate and effective management plan. Diagnostic work-up should always start with a detailed and comprehensive history. As alluded to, recurrent hypoglycaemia can occur with both insulinoma and NICTs. Although the timing of hypoglycaemia can be variable, episodes often occur during the night or early morning before breakfast, when the mitigating effects of food are less likely to occur. Due to the behavioural and appetitive changes induced by recurrent hypoglycaemia, weight gain can also occur with TIH, although the absence of weight gain should not detract from considering such a diagnosis. There may also be specific clinical features such as weight loss that relate to the underlying NICT. Ultimately, however, the diagnosis of TIH and the differentiation between insulinoma and NICT depends on detailed and accurate biochemical testing.
Biochemical investigation
The biochemical investigation of hypoglycaemia depends upon the successful inducement of a hypoglycaemic episode, during which there is analysis of further samples. In the case of TIH, the gold standard test is the 72-hour fast. This usually requires a patient to attend as an in-patient either in a ward-based setting or on a specialist endocrine investigation unit. During such a test, access to water is unlimited, although there is complete restriction of any caloric ingestion, with the aim to induce a hypoglycaemic episode. Occurrence of hypoglycaemia in both insulinoma and NICT is usually characterized clinically by the onset of Whipple’s triad, with associated biochemical evidence (plasma blood glucose level <3.0 mmol/L; 54 mg/dL). In the case of insulinoma, hypoglycaemia occurs in the context of inappropriate hyperinsulinaemia (≥18 pmol/L), associated elevation of serum C-peptide levels (≥0.2 nmol/L) and plasma proinsulin levels (≥5 pmol/L) and suppression of plasma β-hydroxybutyrate levels (≤2.7 mmol/L) (5).
C-peptide is a molecule produced when endogenous proinsulin is cleaved to form insulin, and is secreted from the pancreatic beta-cells in equimolar concentrations to insulin (29). An elevated serum C-peptide level in the context of raised serum levels of proinsulin and insulin indicates the presence of either an insulinoma or factitious use of sulphonylurea drugs (a class of oral glucose-lowering drugs that mainly act by stimulating pancreatic insulin secretion) (29). To diagnose TIH, it is important to exclude exogenous insulin administration as a potential cause of hypoglycaemia through appropriately elevated serum C-peptide levels (circulating C-peptide levels are not affected by exogenous insulin administration), and use of insulin secretagogue therapies (such as sulphonylureas) through either plasma or urine screening for metabolites of sulphonylureas (30).
Distinct from insulinoma, those patients with NICT-related hypoglycaemia manifest hypoinsulinaemia in the context of hypoglycaemia, but with a characteristically increased ratio of serum IGF2-to-IGF1. Suppression of serum IGF1 in patients with NICT occurs due to suppression of GH secretion from increased serum levels of free big-IGF2 (17). This provides an explanation for a raised IGF2-to-IGF1 ratio as a characteristic biochemical signature of NICTs [identifiable via thin-layer liquid chromatography mass spectroscopy (LC/MS)] (31). Patients with NICT-related hypoglycaemia also typically have low serum levels of proinsulin, C-peptide, beta-hydroxybutyrate and IGFBP3 (3,32). However, there is some heterogeneity in the biochemical manifestation of NICT-related hypoglycaemia. We present the characteristic biochemical signatures of TIH during hypoglycaemia in Table 2. Although nominally a 72-hour fasting test, the duration of the test depends on the occurrence of hypoglycaemia (marking its conclusion). In fact, 95% of cases only require 48 hours of fasting to induce hypoglycaemia (with 99% of cases requiring 72 hours) (33). Indeed, there are a few exceptional case reports of insulinoma diagnosis despite a normal 72-hour fasting test (34).

Full table
Immunoassays form the main biochemical diagnostic technique for TIH (35). One important limitation of immunoassays relates to their immunoreactivity against numerous peptides, thereby generating a total concentration of all such peptides in any sample, rather than the concentration of each individual peptide (35). This is relevant to NETs as these tumours may release partially processed pro-hormones that then contribute towards the total measured concentration produced by the assay (35). To address such a challenge, an alternative approach, and one increasingly applied to clinical practice including some cases of NET (glucagonoma and insulinoma) is LC/MS (35), which requires less plasma and represents a cheaper option than current traditional immunoassay-based methods. LC/MS may therefore be a viable future option for application to TIH diagnostics (35). Unfortunately, there is no commercially available immunoassay for big-IGF2 (16,21). Furthermore, the current IGF2 assay may not distinguish between total and free levels of IGF2. This is relevant as NICT-related hypoglycaemia usually associates with increased serum free big-IGF2, although total serum level of IGF2 may not change (21,28,36). Despite these challenges in assaying serum big-IGF2 and IGF2, it is possible to use northern blotting on tumour tissue to detect increased levels of IGF2-mRNA (13,37).
For patients without any occurrence of hypoglycaemia within 48 hours of a fast, premature curtailment of the entire 72-hour fast remains an option (33,38). However, the gold standard test is a 72-hour fast, early termination of which may result in a failure to demonstrate hypoglycaemia (with Whipple’s triad) in a small percentage of patients who are asymptomatic at 48 hours. Furthermore, in those patients with NICTs, it may require a full 72 hours for full beta-cell suppression to occur (33). Most patients undergo admission for a 72-hour fasting test, which does limit its utility and applicability. To mitigate such a challenge, in one study from the US, patients were fasted overnight and then attended an outpatient clinic the next day for hypoglycaemia monitoring. In 40% of patients, this approach was sufficient for a biochemical diagnosis based on the occurrence of hypoglycaemia (patients underwent admission if hypoglycaemia did not then occur in the outpatient setting) (33).
Imaging
Following biochemical confirmation of TIH (with differentiation of insulinoma versus NICT), there is usually a requirement to localize the tumour through focused imaging modalities (11). In one longitudinal study that explored the use of imaging studies for insulinoma introduced into clinical practice until 2007, non-invasive techniques such as transabdominal ultrasound and triple phased spiral CT or MRI (modalities from the 2003–2007 study cohort), enabled the diagnosis of 80% of cases of TIH. Diagnosis of the remaining 20% of cases required the use of invasive techniques, such as Endoscopic Ultrasonography (EUS) or Selective Arterial Calcium Stimulation Testing (SACT) (11). Implementation of such invasive tests reduces the need for blind surgical exploration of insulinomas, with data from the Mayo Clinic showing sensitivity rates of 75% and 93% for EUS and SACT, respectively (11).
Unusual causes of TIH
Although this review focuses on the main causes of TIH (insulinoma and NICT), there are reports from the literature of rare cases of TIH that do not fit into either of these categories. It is important, therefore, to be aware of the potential for such cases when investigating for TIH. In one reported case of a patient with dementia and recurrent falls, there was identification at post-mortem of multiple hepatic lesions that were proinsulin-secreting NETs (39). This case highlights the importance of including measurements of serum proinsulin in the diagnostic work-up of TIH. Of note, there is variable cross-reactivity of proinsulin with the C-peptide assay, dependent upon the specific assay used (39). Although a relatively faster clearance of serum insulin compared to C-peptide can influence the ratio of insulin to C-peptide, in this case the ratio was markedly low, with disproportionately higher levels of serum C-peptide compared to insulin (due to cross-reactivity of proinsulin with the C-peptide assay) (39). Therefore, in cases of markedly elevated serum proinsulin and C-peptide compared with serum insulin, there should be consideration of a diagnosis of proinsulinoma. Whilst the vast majority of cases of proinsulinoma originate within the pancreas, rare cases (such as the one described here) are extra-pancreatic in origin.
Rare cases of NICTs that may present with the biochemical profiles of raised serum IGF2-to-IGF1 ratios from over-production of big-IGF2 from the tumour, include hepatocellular carcinoma (40), pulmonary blastoma (41), and disseminated ductal carcinoma of the breast (42). Furthermore, in rare cases of TIH, a diagnosis of NICT as a cause of recurrent hypoglycaemia may not be apparent from clinical and imaging details, highlighting the importance of careful biochemical assessment. In one reported case of recurrent hypoglycaemia, the underlying cause was NICT originating from multiple pulmonary metastases from a poorly differentiated thyroid carcinoma (43). Biochemistry confirmed an increased serum concentration of big-IGF2, and undetectable levels of serum insulin, IGF1 and GH. Furthermore, on retrospective review, there had been a gradual reduction in the serum concentration of glycated haemoglobin associated with a corresponding increase in the estimated volume of the pulmonary metastases (43).
Other unusual cases of hormonally mediated recurrent hypoglycaemia may not always implicate underlying tumours. In one reported case, a patient with a history of head trauma and reactive hypoglycaemia, developed more frequent and severe hypoglycaemic episodes (44). Following exclusion of TIH, the underlying cause of recurrent hypoglycaemia was severe isolated GH deficiency, which responded well to treatment with recombinant human (rh) GH therapy (44). A report of a rare case of antepartum pituitary failure presenting as recurrent hypoglycaemia and acute onset of headache in a pregnant woman with type 1 DM further highlights the importance of normal pituitary function in the mitigation of recurrent hypoglycaemia (45). A further report describes the potential effects of opioid analgesia on the occurrence of hypoglycaemia resulting from secondary adrenal insufficiency (46). Finally, any disturbance of the central regulation of the hypothalamo-pituitary adrenal and/or somatotropic axes can manifest clinically with recurrent hypoglycaemia (including multiple other clinical features, particularly in children) (47). Therefore, the endocrine diagnostic work-up of recurrent hypoglycaemia should include consideration of pituitary adenoma (including both functioning and non-functioning sub-types) and their prior treatment with surgery and/or radiotherapy, and other hypothalamo-pituitary pathologies (including genetic and/or congenital defects) (47).
The rare and unusual endocrine cases outlined in this sub-section highlight the importance of a thorough biochemical and endocrine assessment in the investigation of recurrent hypoglycaemia, and a preparedness for unusual and rare presentations of TIH.
TIH post-bariatric surgery
A special rare case of TIH (although one shrouded in controversy) may occur as a complication of Roux-en-Y gastric bypass (RYGB) surgery (2). The overall prevalence of hypoglycaemia following RYGB is estimated at 0.2–1% of patients, and is more prevalent in women (2,48). In one case series, patients (n=6) who developed endogenous hyperinsulinaemic hypoglycaemia following RYGB underwent partial pancreatectomies as a treatment strategy. Pathological assessments suggested nesidioblastosis (2,49). Although a subsequent case series revealed similar results (50), further reports have questioned the diagnosis of nesidioblastosis (versus increased nuclear diameter of beta cells) (51), and suggest that post-mortem autolysis may alter cell size and therefore histological appearance (2). Dietary modification represents the first-line of therapy in post-RYGB hypoglycaemia, with a low-carbohydrate diet less likely to cause hypoglycaemia (2,52). Medical therapies for post-RYGB include α-glucosidase inhibitors that reduce the postprandial rise in glucose and insulin, but are limited by their gastrointestinal side-effects (2). Other medical therapeutic strategies include somatostatin analogues that inhibit insulin secretion and reduce gastrointestinal motility, and occasionally diazoxide (53), although there is a lack of evidence for the longer-term efficacy of such therapies (2). Although partial pancreatectomy as a treatment option of post-RYGB surgery has had variable results (54-58), a majority of patients appear to have continuation of their hypoglycaemic symptoms post-pancreatectomy, and therefore this surgical option has been abandoned for the purpose of alleviating hypoglycaemia (2). Finally, surgical reversal of RYGB provides a reasonable option in those patients with severe and refractory hypoglycaemia post-RYGB (2,59).
Management of TIH
A detailed discussion of the management strategies for TIH is beyond the scope of this concise review, and is covered comprehensively elsewhere (2). Here, we only outline the basic principles of management. Due to the rarity of TIH, evidence for its effective management is restricted to case series and individual case reports (16). Broadly, the management of TIH sub-divides into two main strategies: (I) surgical excision or ablation of the underlying insulinoma or NICT, and; (II) medical and lifestyle management of TIH-associated hypoglycaemia.
Surgical excision
Where possible, there should be complete surgical resection of an insulinoma or NICT causing hypoglycaemia, and if successful, this often cures further hypoglycaemic episodes (16). Surgical excision of insulinoma may not be possible in the context of smaller tumours that are undetectable on imaging techniques. Furthermore, complete surgical resection of some NICT tumours may not be possible due to their size and accessibility, especially in the context of metastases (16). Surgical resection allows for immunohistochemical confirmation of tumour sub-type (60). In cases of NICTs where surgical excision is not possible, ablative measures are an option (60). Selective embolization and radiofrequency ablation can help in the alleviation of hypoglycaemia (13). However, rapid necrosis of the tumour that can occur during such procedures can result in an increase in circulating levels of big-IGF2, manifesting in further hypoglycaemia. Therefore, caution is required during ablation and embolization procedures for NICTs (13).
Medical and lifestyle management
In patients who present acutely with recurrent hypoglycaemia relating to TIH, it is important to initiate prompt management with intravenous hydration and glucose infusions (13). In the outpatient setting, and prior to any surgery for TIH, there is usually provision of dietary advice to patients, including frequent small meals to reduce the frequency of hypoglycaemia.
Medical therapy for insulinomas includes administration of diazoxide (200–600 mg per day, orally in divided doses), with the dose gradually titrated to alleviate symptoms (9). Somatostatin analogues represent an alternative medical therapy, although insulinomas variably express somatostatin receptors (9,61). Overall, use of diazoxide or somatostatin analogues control the hypoglycaemic symptoms of insulinoma in 50–60% of patients (5,62,63). Glucocorticoids are the most documented medical treatment to prevent hypoglycaemia. As a bridge to surgery, high doses of glucocorticoids are used (equivalent to prednisolone 30–60 mg per day) (16,64). Indeed, a useful illustration of the effectiveness of high-dose glucocorticoids as a treatment option in this context is the reported prevention of hypoglycaemia from its use as a monotherapy (16,64). However, some cases of TIH are resistant to even very high doses of glucocorticoids, in which recurrent hypoglycaemia can persist (16).
Regarding the effective management of TIH from NICT, the treatment of choice is supra-physiological doses rhGH, at 3–12 mg per day (16,65). The efficacy of rhGH occurs via several mechanisms that include increased levels of IGF1, IGFBP3 and suppression of the peripheral uptake of glucose (with an overview in Figure 1) (16,65,66). Furthermore, administration of glucocorticoid therapies in patients with TIH from NICTs also decrease serum levels of IGF2. Use of glucocorticoid and rhGH therapies can enable palliative weaning off intravenous glucose in some patients with NICT-related hypoglycaemia (13). However, neither rhGH nor glucocorticoid therapies have undergone randomised trials. Furthermore, there remains a theoretical possibility of rhGH-induced stimulation of tumour cells when used in patients with NICTs (16). We provide a summary of the therapeutic options for insulinoma and NICT-induced hypoglycaemia in Table 1.
Future directions
Most rare conditions suffer from a relative lack of high-quality evidence that stems from randomised controlled trials. By definition, a rare condition simply lacks enough cases to power adequately any meaningful placebo-controlled trial. This poses a problem for conditions such as TIH. Instead of a reliable and robust evidence-base, our practice in the investigation and management of such rare conditions usually rests on case series, case reports and expert opinion. Often, clinical management of such rare conditions remains constant over decades with each new generation of healthcare professional adopting the accepted practice of their immediate senior colleagues. Such an approach is entirely understandable in the face of deficient high-quality evidence. However, we should still question our approach towards the investigation and management of rare conditions, and not simply accept that current approaches are necessarily the best just because no one has done it differently. In this context, TIH presents some specific challenges, outlined here, that need to be addressed in future clinical practice:
Lack of clinical awareness
As with many rare conditions, TIH suffers from a broader lack of awareness amongst healthcare professionals in general. Many non-specialist colleagues may never encounter a patient with TIH during their entire career. This perhaps explains why there can be a delay in the diagnosis of TIH, through a lack of clinical suspicion and timely referral due to clinical inexperience and reduced awareness. Despite its rarity though, TIH is nonetheless a condition that demands accurate and timely diagnosis and effective individualised management strategies, given the implications of unrecognised and untreated recurrent hypoglycaemia for the patient and for society in general (including road safety for example). Moreover, as hypoglycaemia is potentially fatal, it is important that healthcare professionals receive proper education regarding the key clinical features of hypoglycaemia, and the possibility of underlying TIH. Raising awareness of this important condition amongst colleagues could save lives. Since specialist investigation and management is required, it is also essential that there is prompt referral of any patient with a clinical suspicion of TIH to an Endocrine specialist colleague.
Diagnostic challenges
Investigation of recurrent hypoglycaemia presents specific challenges given the need to provoke a hypoglycaemic episode. This usually requires in-patient admission, which is often difficult given the extreme pressure on hospital beds in our modern-day era. Furthermore, patients may be reluctant to come into a hospital for a 3-day fasting test for many reasons. As outlined earlier, one alternative would be for patients to attend an outpatient investigation unit for one day following an overnight fast, thereby avoiding the need for hospital admission in at least a subgroup of patients. However, even with this approach, many patients will subsequently require hospital admission (33). Further challenges include the timing of samples: regular monitoring of blood glucose is required, and many samples (including serum and urine) need sending to the biochemistry lab concurrently and during a hypoglycaemic episode. Given the extreme pressures on ward-based staff, particularly during busy periods, it can be difficult to adhere to a strict protocol that is utterly dependent upon close observation and impeccable timing. Finally, even if a hypoglycaemic episode is provoked and all samples timed correctly, the interpretation of biochemical and endocrine data can pose its own challenges. Ambiguity within the biochemical data often prompts a repeat prolonged fasting test.
It is not easy to overcome the diagnostic challenges of TIH outlined here. Provocation of hypoglycaemia carries its own risks. This should only occur within a medical setting with appropriately trained staff, equipment and therapies. Accurate biomarkers of insulinoma and NICTs (through urinary metabolomics assessment for example) represents a possible future alternative diagnostic strategy to a prolonged fasting test. This could potentially avert the need to provoke hypoglycaemia. This should form a focus for future research: in theory, a reliable metabolomics signature of TIH sub-type should be possible, but will require much research in multiple cohorts and settings to show reliability, accuracy and clinical utility.
Management challenges
There are multiple challenges associated with the management of TIH. Underlying tumours (primarily insulinomas and occasionally NICTs) may be relatively small and therefore difficult to identify on imaging. In such cases, surgical resection may not be possible. Furthermore, in cases of loco-regional spread and/or metastases (rare with insulinoma, but commoner with NICTs), surgery may not be indicated or effective at removing the underlying cause of hypoglycaemia. Regarding medical therapies, sourcing relatively high doses of rhGH semi-urgently can pose its own challenges, especially if a specific funding request is required. Finally, patients and their relatives may find living with recurrent hypoglycaemia stressful. The need for lifestyle modification and regular glucose monitoring can be challenging, particularly in the context of underlying malignancy. For these reasons, patients with TIH may require additional psychological support.
Conclusions
To conclude, TIH is a rare form of recurrent hypoglycaemia stemming usually from underlying insulinoma or a NICT, that has important and potentially fatal implications for the patient. Although infrequently encountered outside of the Endocrine realm, in our view all healthcare professionals should be aware of TIH as a clinical entity. Such raised awareness amongst healthcare colleagues would help to avoid missed or delayed diagnoses, and facilitate prompt referral for focused Endocrine investigations. TIH demands accurate diagnosis given the diagnosis-dependent and individualised specific approaches to management. Where possible, we should usually strive for surgical resection of the underlying tumour in TIH, with such an approach offering the possibility of lifelong cure. Unfortunately, however, surgical resection is not always possible, and long-term medical therapies may be required. Although the diagnosis of TIH usually requires admission for a prolonged fasting test, future research should focus on alternate investigational routes to diagnosis, given the inherent challenges and potential safety concerns that an in-patient prolonged fasting test raises. Options include the possibility of recognition of specific metabolomics signatures of insulinoma and NICT, which if reliable and accurate enough, could even ultimately replace the prolonged fasting test for the future diagnosis of TIH and its subtypes. Future studies should also focus on the development of novel therapies for TIH, including proper assessment of the efficacy and safety of existing therapies (such as rhGH for NICT-induced TIH). Finally, we should always remember that behind every case of TIH there is a patient, with a family, loved ones and inner and outer social circles. Recurrent hypoglycaemia affects patients’ lives and potentially the lives of many others. Therefore, compassion and understanding of the human aspects of recurrent hypoglycaemia are also integral to the successful management of TIH.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rousseau Gama) for the series “Adult Spontaneous Hypoglycaemia” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/jlpm-20-110
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jlpm-20-110). The series “Adult Spontaneous Hypoglycaemia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iqbal A, Heller S. Managing hypoglycaemia. Best Pract Res Clin Endocrinol Metab 2016;30:413-30. [Crossref] [PubMed]
- Kittah NE, Vella A. Management of endocrine disease: pathogenesis and management of hypoglycemia. Eur J Endocrinol 2017;177:R37-47. [Crossref] [PubMed]
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709-28. [Crossref] [PubMed]
- Wilder RM, Allan FN, Power MH, et al. Carcinoma of the islands of the pancreas: hyperinsulinism and hypoglycemia. JAMA 1927;89:348-55. [Crossref]
- Iglesias P, Díez JJ. Management of endocrine disease: a clinical update on tumor-induced hypoglycemia. Eur J Endocrinol 2014;170:R147-57. [Crossref] [PubMed]
- Service FJ, McMahon MM, O'Brien PC, et al. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991;66:711-9. [Crossref] [PubMed]
- Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg 1935;101:1299-335. [Crossref] [PubMed]
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. [Crossref] [PubMed]
- Mathur A, Gorden P, Libutti SK. Insulinoma. Surg Clin North Am 2009;89:1105-21. [Crossref] [PubMed]
- Boukhman MP, Karam JH, Shaver J, et al. Insulinoma--experience from 1950 to 1995. West J Med 1998;169:98-104. [PubMed]
- Placzkowski KA, Vella A, Thompson GB, et al. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab 2009;94:1069-73. [Crossref] [PubMed]
- Dizon AM, Kowalyk S, Hoogwerf BJ. Neuroglycopenic and other symptoms in patients with insulinomas. Am J Med 1999;106:307-10. [Crossref] [PubMed]
- Singhal A, Hadi R, Mehrotra K, et al. Paraneoplastic Hypoglycaemia: A Rare Manifestation of Pelvic Gastrointestinal Stromal Tumour. J Clin Diagn Res 2017;11:XD01-2. [Crossref] [PubMed]
- Escobar GA, Robinson WA, Nydam TL, et al. Severe paraneoplastic hypoglycemia in a patient with a gastrointestinal stromal tumor with an exon 9 mutation: a case report. BMC Cancer 2007;7:13. [Crossref] [PubMed]
- Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev 1991;7:79-91. [Crossref] [PubMed]
- Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab 2014;99:713-22. [Crossref] [PubMed]
- Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res 2006;16:211-6. [Crossref] [PubMed]
- Ahluwalia N, Attia R, Green A, et al. Doege-Potter Syndrome. Ann R Coll Surg Engl 2015;97:e105-7. [Crossref] [PubMed]
- Trivedi N, Mithal A, Sharma AK, et al. Non-islet cell tumour induced hypoglycaemia with acromegaloid facial and acral swelling. Clin Endocrinol (Oxf) 1995;42:433-5. [Crossref] [PubMed]
- de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer 2007;14:979-93. [Crossref] [PubMed]
- Schovanek J, Cibickova L, Ctvrtlik F, et al. Hypoglycemia as a Symptom of Neoplastic Disease, with a focus on Insulin-like Growth Factors Producing Tumors. J Cancer 2019;10:6475-80. [Crossref] [PubMed]
- Frysak Z, Schovanek J, Iacobone M, et al. Insulin-like Growth Factors in a clinical setting: Review of IGF-I. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:347-51. [Crossref] [PubMed]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer 2004;4:505-18. [Crossref] [PubMed]
- Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem 1978;253:2769-76. [Crossref] [PubMed]
- Rinderknecht E, Humbel RE. Primary structure of human insulin-like growth factor II. FEBS Lett 1978;89:283-6. [Crossref] [PubMed]
- Hizuka N, Fukuda I, Takano K, et al. Serum high molecular weight form of insulin-like growth factor II from patients with non-islet cell tumor hypoglycemia is O-glycosylated. J Clin Endocrinol Metab 1998;83:2875-7. [PubMed]
- Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med 1997;336:633-40. [Crossref] [PubMed]
- Frystyk J, Skjaerbaek C, Zapf J, et al. Increased levels of circulating free insulin-like growth factors in patients with non-islet cell tumour hypoglycaemia. Diabetologia 1998;41:589-94. [Crossref] [PubMed]
- DeWitt CR, Heard K, Waksman JC. Insulin & C-peptide levels in sulfonylurea-induced hypoglycemia: a systematic review. J Med Toxicol 2007;3:107-18. [Crossref] [PubMed]
- Kandaswamy L, Raghavan R, Pappachan JM. Spontaneous hypoglycemia: diagnostic evaluation and management. Endocrine 2016;53:47-57. [Crossref] [PubMed]
- Miraki-Moud F, Grossman AB, Besser M, et al. A rapid method for analyzing serum pro-insulin-like growth factor-II in patients with non-islet cell tumor hypoglycemia. J Clin Endocrinol Metab 2005;90:3819-23. [Crossref] [PubMed]
- Pink D, Schoeler D, Lindner T, et al. Severe hypoglycemia caused by paraneoplastic production of IGF-II in patients with advanced gastrointestinal stromal tumors: a report of two cases. J Clin Oncol 2005;23:6809-11. [Crossref] [PubMed]
- Service FJ, Natt N. The prolonged fast. J Clin Endocrinol Metab 2000;85:3973-4. [Crossref] [PubMed]
- Soh AW, Kek PC. Insulinoma in a patient with normal results from prolonged fast and glucagon-induced hypoglycemia. Endocr Pract 2010;16:838-41. [Crossref] [PubMed]
- Kay RG, Challis BG, Casey RT, et al. Peptidomic analysis of endogenous plasma peptides from patients with pancreatic neuroendocrine tumours. Rapid Commun Mass Spectrom 2018;32:1414-24. [Crossref] [PubMed]
- Hoekman K, van Doorn J, Gloudemans T, et al. Hypoglycaemia associated with the production of insulin-like growth factor II and insulin-like growth factor binding protein 6 by a haemangiopericytoma. Clin Endocrinol (Oxf) 1999;51:247-53. [Crossref] [PubMed]
- Daughaday WH, Emanuele MA, Brooks MH, et al. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med 1988;319:1434-40. [Crossref] [PubMed]
- Hirshberg B, Livi A, Bartlett DL, et al. Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab 2000;85:3222-6. [Crossref] [PubMed]
- Cheah SK, Halsall D, Barker P, et al. Refractory spontaneous hypoglycaemia: a diagnostic conundrum. Endocrinol Diabetes Metab Case Rep 2018;2018:18-0049. [Crossref] [PubMed]
- Rana P, Kim B. A Unique Case of IGF-2 Induced Hypoglycemia Associated with Hepatocellular Carcinoma. Case Rep Endocrinol 2019;2019:4601484 [Crossref] [PubMed]
- Mohajeri Tehrani MR, Ghorbani Abdehgah A, Molavi B, et al. Pulmonary blastoma: a case report and brief review of the literature of tumor-induced hypoglycemia. J Diabetes Metab Disord 2016;15:32. [Crossref] [PubMed]
- Bessell EM, Selby C, Ellis IO. Severe hypoglycaemia caused by raised insulin-like growth factor II in disseminated breast cancer. J Clin Pathol 1999;52:780-1. [Crossref] [PubMed]
- Morioka T, Ohba K, Morita H, et al. Non-islet cell tumor-induced hypoglycemia associated with macronodular pulmonary metastases from poorly differentiated thyroid carcinoma. Thyroid 2014;24:395-9. [Crossref] [PubMed]
- Pia A, Piovesan A, Tassone F, et al. A rare case of adulthood-onset growth hormone deficiency presenting as sporadic, symptomatic hypoglycemia. J Endocrinol Invest 2004;27:1060-4. [Crossref] [PubMed]
- Tahrani AA, West TE, Macleod AF. An unusual cause of severe hypoglycaemia in type 1 diabetes mellitus. Antepartum pituitary failure: a case report and literature review. Exp Clin Endocrinol Diabetes 2007;115:136-8. [Crossref] [PubMed]
- Tabet EJ, Clarke AJ, Twigg SM. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep 2019;12:230551 [Crossref] [PubMed]
- Patti G, Guzzeti C, Di Iorgi N, et al. Central adrenal insufficiency in children and adolescents. Best Pract Res Clin Endocrinol Metab 2018;32:425-44. [Crossref] [PubMed]
- Marsk R, Jonas E, Rasmussen F, et al. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia 2010;53:2307-11. [Crossref] [PubMed]
- Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005;353:249-54. [Crossref] [PubMed]
- Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 2005;48:2236-40. [Crossref] [PubMed]
- Meier JJ, Butler AE, Galasso R, et al. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care 2006;29:1554-9. [Crossref] [PubMed]
- Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis 2008;4:492-9. [Crossref] [PubMed]
- Gonzalez-Gonzalez A, Delgado M, Fraga-Fuentes MD. Use of diazoxide in management of severe postprandial hypoglycemia in patient after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2013;9:e18-9. [Crossref] [PubMed]
- Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc 2010;24:2547-55. [Crossref] [PubMed]
- Alvarez GC, Faria EN, Beck M, et al. Laparoscopic spleen-preserving distal pancreatectomy as treatment for nesidioblastosis after gastric bypass surgery. Obes Surg 2007;17:550-2. [Crossref] [PubMed]
- Thompson GB, Service FJ, Andrews JC, et al. Noninsulinoma pancreatogenous hypoglycemia syndrome: an update in 10 surgically treated patients. Surgery 2000;128:937-44; discussion 944-5. [Crossref] [PubMed]
- Harness JK, Geelhoed GW, Thompson NW, et al. Nesidioblastosis in adults. A surgical dilemma. Arch Surg 1981;116:575-80. [Crossref] [PubMed]
- Vanderveen KA, Grant CS, Thompson GB, et al. Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery 2010;148:1237-45; discussion 1245-6. [Crossref] [PubMed]
- Campos GM, Ziemelis M, Paparodis R, et al. Laparoscopic reversal of Roux-en-Y gastric bypass: technique and utility for treatment of endocrine complications. Surg Obes Relat Dis 2014;10:36-43. [Crossref] [PubMed]
- Mele C, Brunani A, Damascelli B, et al. Non-surgical ablative therapies for inoperable benign insulinoma. J Endocrinol Invest 2018;41:153-62. [Crossref] [PubMed]
- Arnold R, Wied M, Behr TH. Somatostatin analogues in the treatment of endocrine tumors of the gastrointestinal tract. Expert Opin Pharmacother 2002;3:643-56. [Crossref] [PubMed]
- Abboud B, Boujaoude J. Occult sporadic insulinoma: localization and surgical strategy. World J Gastroenterol 2008;14:657-65. [Crossref] [PubMed]
- Tucker ON, Crotty PL, Conlon KC. The management of insulinoma. Br J Surg 2006;93:264-75. [Crossref] [PubMed]
- Teale JD, Wark G. The effectiveness of different treatment options for non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) 2004;60:457-60. [Crossref] [PubMed]
- Teale JD, Blum WF, Marks V. Alleviation of non-islet cell tumour hypoglycaemia by growth hormone therapy is associated with changes in IGF binding protein-3. Ann Clin Biochem 1992;29:314-23. [Crossref] [PubMed]
- Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol (Oxf) 1998;49:491-8. [Crossref] [PubMed]
Cite this article as: Barber TM, Bannon CAM, Weickert MO, Kyrou I, Randeva HS. Tumour-induced hypoglycaemia: a narrative review. J Lab Precis Med 2021;6:22.


