
Small variations in fasting blood glucose have significant effects in diagnosis of gestational diabetes mellitus
Introduction
Gestational diabetes mellitus (GDM) is the most common complication associated with pregnancy (1). This temporary condition may appear during the pregnancy and disappear after the delivery (2). The diagnosis of GDM depends upon fasting blood glucose (FG) levels and an oral glucose tolerance test (OGTT) (3,4).
The Hyperglycemia Adverse Pregnancy Outcome (HAPO) study was the first to derive GDM diagnosis cutoff values for FG and OGTT at 24 and 35 weeks of gestation based on the risk of developing perinatal complications (5), such as macrosomia, cesarean delivery, neonatal hypoglycemia, hyperinsulinemia (C-peptide in the cord). Table 1 shows the FG and OGTT cutoffs for diagnosing GDM for different values of the odds ratio (OR =1.5, 1.75, or 2.0) of developing perinatal complications (7,8). The conclusions of the HAPO study were endorsed by the International Association of Diabetes in Pregnancy Study Groups (IADPSG) (6) and different worldwide guidelines for GDM, including the American Diabetes Association (ADA) and the Brazilian Diabetes Society (SBD) (3,4) implement the HAPO study recommendations for diagnosing GDM at the third trimester.
Table 1
| Sample time | Threshold values for glycemia | ||
|---|---|---|---|
| 1.50 [OR] | 1.75 [OR] | 2.0 [OR] | |
| Fasting plasma glucose | 5.0 [90] | 5.1 [92] | 5.3 [95] |
| 1-hour plasma glucose | 9.3 [167] | 10.0 [180] | 10.6 [191] |
| 2-hour plasma glucose | 7.9 [142] | 8.5 [153] | 9.0 [162] |
OR for OGTT, with 75 g glucose load, during the 24–32 weeks of gestation. Thresholds are plasma glucose concentrations in mmol/L (mg/dL) for odds ratio for increased neonatal body fat, large for gestational age and cord serum C-peptide more than 90th centile. The odds ratio and glycemic cut-off were from HAPO study with diagnostic criteria of only one point above the cut-off. Highlighted the column with the OR 1.75, cut-off applied in the most global guidelines. Adapted from Constan et al. (5) and IADPSG (6). OR, odds ratio; OGTT, oral glucose tolerance test; HAPO, Hyperglycemia Adverse Pregnancy Outcome study; IADPSG, International Association of Diabetes in Pregnancy Study Groups.
In contrast, there is not a consensus regarding the universal screening of GDM at the beginning of pregnancy (<12 weeks). Positions for and against screening are supported by different studies (9,10). GDM cases ascertained in the first trimester of pregnancy using the cut-off on FG ≥5.1 mmol/L proposed by the IADPSG Panel do not have a perfect concordance with GDM cases ascertained using FG and OGTT measured at the gestational window between 24–28 weeks (11). Nevertheless, the risks to pregnant women and the fetus, identified by screening with fasting glucose at the beginning of pregnancy are relevant. Consequently, societies such as the Brazilian Diabetes Society (SBD), among others, do recommend this screening (4). The SBD suggests the isolated use of fasting glucose for the diagnosis of GDM in situations where financial and technical feasibility make the recommendations for diagnosing GDM in its entirety impossible.
In this study, we evaluated sources of variation for the diagnostic criteria of GDM with FG, including the effect resulting from the variation of the prevalence of GDM with changes of a few milligrams from the recommended the cut-off criterion (5.1 mmol/L).
We present the following article in accordance with the MDAR reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-57/rc).
Methods
Sample
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Federal University of Parana’s Ethics Committee (CAAE: 39460414.0.0000.0102) and individual consent for this retrospective analysis was waived.
De-identified registers of fasting glycemia were obtained from the Laboratório Municipal de Curitiba, Parana State, South of Brazil. Register data (collected from 2016 to 2020) form early pregnancy (n=38,489) were characterized by less than 12 weeks of gestation (4–12 weeks of gestation; median 7 weeks). Data from a second group were captured from a fasting glycemia (n=60,432) of the OGTT performed at 24–28 weeks of gestation. No clinical information was obtained for the studied sample population.
For early and late pregnancies groups, data with a plasma glycemia less than 2.5 mmol/L (45 mg/dL) or more than 5.8 mmol/L (105 mg/dL) were removed. The same exclusion criteria were applied in the HAPO study (8). GDM criteria was established according to ADA (3) and SBD (4).
Glucose measurement
Fasting glycemia was measured in plasma (NaF-EDTA, BD vacutainer) with hexokinase UV. The measurement was automated in Cobas C502 (Roche Diagnostics) with reagent, calibrator, and controls provided by de manufacturer. The interassay mean coefficient of analytical variation (CVa) was 2.0% (range, 1.5–2.2%).
Analytical and biological variation
The analytical (CVa) and intraindividual (within-subject) biological (CVi) variation, were presented as a coefficient of variation [CV = (standard deviation/mean) ×100]. CVa, was calculated as mean value from the interassay (day-by-day) quality control of the “normal” Precinorm U (Roche Diagnostics) which had a mean of 5.17 mmol/L (93 mg/dL) concentration; for a period superior to 6 months (12). CVi, was defined as a random fluctuation of an analyte (glucose) around a homeostatic point, as described by Frazer (13).
The combined effect of CVa and CVi was calculated by CVtotal = (CVa2 + CVi2)1/2 according to Braga and Panteghini (14).
The reference change values (RCVs), were calculated by the equation: RCV =21/2 × Zp × (CVa2+ CVi2)1/2. The selected Zp was 1.65 (unidirectional 95% z-score for a P<0.05) which resulted in the equation RCV =2.33 × (CVa2 + CVi2)1/2, as recommended by Frazer (15).
Statistical analysis
Descriptive statistics, normality (Kolmogorov-Smirnov), frequencies, and 95% confidence intervals (95% CIs) were calculated with the software MedCalc Statistical Software version 19.1 (MedCalc Software bv, Ostend, Belgium). A probability less than 5% (P<0.05) was considered significant.
Results
The characteristics of the study groups are presented in Table 2. The register of gestation in early (<12 weeks) and late (24–28 weeks) showed a median of 25 years old for both groups and a fasting glycemia of 4.5 and 4.3 mmol/L, respectively. Age and fasting glycemia were not different among the groups (P>0.05; Mann-Whitney U test).
Table 2
| Parameters | Gestational period (weeks of gestation) | |
|---|---|---|
| Early (<12 weeks) | Late (24–28 weeks) | |
| Sample size, n | 38,489 | 60,432 |
| Age, years | 25.0 [21–30] | 25.0 [21–30] |
| Fasting glycemia, mmol/L | 4.5 (4.2–4.8) | 4.3 (4.1–4.6) |
Values were median and interquartile range (25th–75th). Age and fasting glycemia showed non-normal distribution (tested by Kolmogorov-Smirnov test, P<0.001).
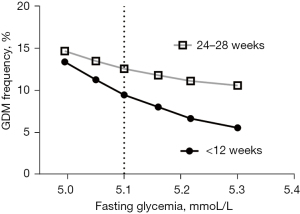
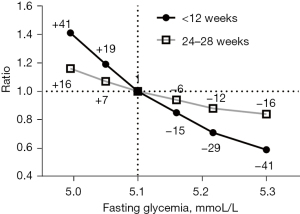
A frequency GDM simulation and comparison of according to different cut-off values were showed in Table 3, as well as Figures 1,2 for early and late stages of pregnancy. The cut-off 5.1 mmol/L, accepted as GDM diagnostic in most guidelines are considered a reference. The others cutoff selected were in the range observed for different Odds Ratio risk for GDM in HAPO study as presented in Table 1.
Table 3
| No. | FBG cut-off | <12 weeks (n=38,489) | 24–28 weeks (n=60,432) | |||||
|---|---|---|---|---|---|---|---|---|
| mg/dL | mmol/L | GDM% (95% CI) | Ratio | GDM% (95% CI) | Ratio | |||
| 1 | 90 | 4.99 | 13.4 (12.5–14.4) | 1.41 (+41%) | 14.7 (13.9–15.5) | 1.16 (+16) | ||
| 2 | 91 | 5.05 | 11.3 (10.4–12.3) | 1.19 (+19) | 13.5 (12.8–14.3) | 1.07 (+7) | ||
| 3 | 92 | 5.10 | 9.5 (8.6–10.5) | 1.0 (reference) | 12.6 (11.8–13.4) | 1.0 (reference) | ||
| 4 | 93 | 5.16 | 8.1 (7.1–9.1) | 0.85 (−15) | 11.8 (11.1–12.6) | 0.94 (−6) | ||
| 5 | 94 | 5.22 | 6.7 (5.7–7.7) | 0.71 (−29) | 11.2 (10.4–11.9) | 0.88 (−12) | ||
| 6 | 95 | 5.27 | 5.6 (4.7–6.7) | 0.59 (−41) | 10.6 (9.9–11.4) | 0.84 (−16) | ||
Highlight the concentration of cut-off for GDM in current guidelines 5.1 mmol/L (92 mg/dL) ratio calculation. For <12 weeks of gestation, ratio reference 1.0 is 9.50%; ratio = GDM/9.50; percentage (%) compared to reference =100(GDM freq%/9.50%)−100. For 24−28 weeks of gestation, ratio 1 is 12.6%; ratio = GDM/12.50; percentage (%) compared to reference =100 (GDM freq%/12.6%)−100. Positive and negative percentages were calculate comparing the increased or decreased the frequencies of estimated GDM comparing to 5.1 mmol/L frequencies (reference) or 100%. FBG, fasting blood glucose; GDM, gestational diabetes mellitus; 95% CI, 95% confidence interval.
The variability in fasting glycemia imposed by analytical and biological variation were presented in Figure 3. The selected CVa (2.0%) was observed in our internal quality control and consistent with the reagent and automation system used. The CVi estimation (5.0%) were obtained in literature from Ricós et al. (16). The combine CVa + CVi calculated as (2.02+5.02)1/2 showed an estimated variation of 5.4%.
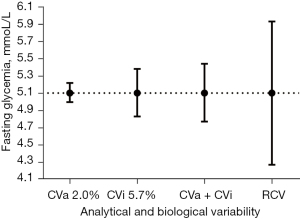
The RCVs were calculated with the equation as showed in Material and Methods, RCV =2.33×(2.02+5.02)1/2, which results in an estimated variation of 12.5%.
When the described coefficients of variation and RCV were applied at the concentration of 5.1 mmol/L (92 mg/dL), the 95% distribution interval (mmol/L) were CVa 2% (4.99–5.21); CVi 5% (4.83–5.38); CVa + CVi (4.77–5.44) and RCV 12.5% (4.27–5.93), as showed in Figure 3.
Discussion
In the study, we have shown the impact of changes of a few millimoles (or milligrams) from the recommended cut-off criterion (5.1 mmol/L) over the prevalence of GDM in early and late stages of pregnancy (Figures 1,2) and the effect of the analytical and biological variations (Figure 3), as well.
We applied cut-off values for fasting glycemia concentrations between 5.0 to 5.3 mmol/L, in a large sample size register of pregnant women, in early (<12 weeks) and late (24–28 weeks) stages of pregnancy. The selected cut-off values emulated the observed in HAPO study for odds ratio 1.5 to 2.0 of risk for complications associated with GDM, as showed in Table 1. The frequencies of pregnant women above the cut-off, represents hypothetically gestational diabetes subjects (Figure 1). As expected, the modification in the cut-off value for fasting glycemia resulted in a change of frequency for GDM. In late stages of pregnancy (24–28 weeks), the variation of fasting glycemia from 5.0 to 5.3 mmol/L resulted in modifications of the respective estimated of GDM frequencies: 14.7% to 10.6%, a variation of 4.1 percentage points. For the same range of fasting glycemia, the estimated GDM frequencies were 13.4% to 5.6% in early stages of pregnancy, a variation of 7.8 percentage points.
A detailed of estimated GDM frequencies according to cut-off values (Figure 2), showed that the variation in early stages of pregnancy were about 50% higher when compared to the late stage. The 0.055 mmol/L (1 mg/dL) variation in fasting glycemia in the cut-off criterion of 5.1 mmol/L (HAPO criterion) promotes a variation in the estimated frequency of GDM in about 7% (+7% to −6%) of late-stage cases and about 15% (+19 to −15%) near the beginning of pregnancy.
We additionally, evaluated the theoretical effect of the analytical and biological variations on the cut-off concentration (5.1 mmol/L) for the diagnosis of GDM (Figure 3).
The analytical variability recommended for blood glucose, in an automated system, has been reported as a CVa ≤2.2% (17). CVa, represents the magnitude of the glucose assay reproduction or daily imprecision. The CVa variation reported for automated assays systems were between 1.5% to 3.4% (18). In our study, CVa for glycemia was 1.5 to 2.2% (mean 2.0%), for a “normal” control serum with a glucose concentration of about 5.0 mmol/L.
Considering a CV of 2%, the simple repetition of a serum/plasma sample with glucose concentrations between 5.0 and 5.2 mmol/L are expected with a 95% probability for an average cut-off value of 5.1 mmol/L. Therefore, repeating a sample that presents criteria for GDM at the cut-off limit, presents a chance of about 50% of the given patient not being diagnosed (concentrations 5.0 and 5.05 mmol/L). In addition, the 2% CVa, is reached by laboratories with automated systems and effective quality control. This pattern may not be followed by laboratories with less precise systems, increasing the variability substantially.
The influence of analytical performance is more critical when diagnostic decisions are based on decision limits such as IADPSG-HAPO which are endorsed in several medical guidelines (19).
Concerning CVa, a discussion is warranted regarding the assumption the optimized pre-analytical condition (20). The “glycolytic effect,” the blood cells consumption of glucose, might produce a change in glycemia of about 0.55 mmol/L (10 mg/dL) per hour if the sample was not treated with antiglycolytic agents, such as sodium fluoride (21).
For the study, we selected a revised intraindividual variation coefficient (CVi, 5.0%; 95% CI, 4.1–12.0) for fasting glycemia, consolidated by meta-analysis (16). CVi, captures the mean glucose variation, which results from factor such as growing, gender, hormonal cycles, diet, physical exercises, among others as described by Ricós et al. (16).
Fixing the set point at 5.1 mmol/L and applying a 5% CVi, fasting glucose with 95% probability, the resulting interval was 4.8 to 5.4 mmol/L, as observed in Figure 3. It is expected that a cut-off value of 5.1 mmol/L is appropriate for pregnant women with an average CVi of 5%, and a fasting glycemia in the range of 4.8 to 5.4 mmol/L.
Combining the effects of CVa 2% and CVi 5% (CVa + CVi), for FG, with the statistical treatment described in materials and methods, it is observed that the variation in blood glucose is essentially associated with CVi alone (Figure 3). This effect is expected when the CVa/CVi ratio is less than 0.5 (13). In the study, the observed ratio was 0.4 (2%/5%; CVa and CVi), was not optimum, but classified as “desirable” or acceptable (22).
The effect of analytical (CVa) and biological (CVi) variation on GDM prevalence is important. Agarwal et al. (2015) (23), applying the concept of total analytical error (TAEL) defined as TAEL =1.65 CVa + Bias (up to glycemia 9.0 mmol/L; CVa =2% and Bias =1.7%; TAEL =5%), on glycemia measurements, showed that GDM prevalence can vary 0.5–2.0 times the reported prevalence.
The RCV or critical difference, as presented, is a way of identifying change resulting from a second sequential dosage of the analyte (fasting glycemia). Applying the Z statistic to the biological and analytical variability, it is possible to establish with 95% confidence whether the second dosage is higher or lower than the first (15,24).
Applying the equation described for RCV (with CVa 2% and CVi 5%) to a set point of 5.1 mmol/L, a subsequent second FG measurement would pertain to a different distribution, to a 95% probability, if it were less than 4.3 mmol/L or greater than 5.9 mmol/L (Figure 3).
Many criticisms of the use of RCV are described (25-28). Among these, two deserve mention. First, RCV estimates are consistent if the analysis is for an individual with a CVi close to the average (CVi 5% in the study), used in the calculation (29). Secondly, the CVi is estimated for healthy individuals, which can be significantly modified in the face of pathological processes (30). In a study with a small sample size, Nigam et al. (31) showed that glycemic variability (GV) was higher at 24–36 weeks of gestation for GDM patients compared to healthy pregnant and nonpregnant women.
Considering the magnitude of the fasting glycemia variation presented, the study reinforces the need to repeat the test to confirm the diagnosis, when fasting glycemia in a pregnant woman presents concentrations close to the cutoff value (5.1 mmol/L).
With the RCV estimate, pregnant women with a blood glucose ≥4.4 to 5.05 mmol/L could not be characterized as low risk for GDM in screening or diagnosis based in a cutoff point of 5.1 mmol/L.
Trujillo et al. (32) analyzed OGTT in a cohort of 5,564 pregnant women at 20–28 weeks of gestation. These authors reported that based on fasting glycemia the probability of having GDM was very low (~2%). This was at 20–28 weeks of gestation with cut-offs bellow 4.4 and 4.7 mmol/L. These results agree of our theorical analysis, which suggest 4.4 mmol/L might be an important decision value. Fasting glycemia below 4.4 mmol/L suggested a low risk for GDM. At a value above this cut-off, between 4.4 to 5.05 mmol/L, the test should be repeated.
In addition, blood glucose repetitions should be more frequent at the beginning of pregnancy, where small variations in glucose concentration, such as 0.055 mmol/L (1 mg/dL), modify the prevalence of GDM more significantly, when compared to the fasting glycemia in OGTT at 24–28 weeks of gestation.
GV and its relationship with gestational diabetes has been intensively investigated with controversial results (33). Long-term or gestational studies of GV have shown interest as a risk marker for GDM (34,35). In general, it was stablished that pregnant women with GDM have higher GV compared to normal pregnant women (36).
Wei et al. [2018] (37) suggest that women of childbearing age may benefit from early detection of GDM by monitoring the pattern of GV, which high GV has been associated with increased risk for GDM and large of gestational age (LGA) fetuses.
Our study assesses and magnifies the variability of FG during pregnancy, in analytical and biological terms, and may contribute to an adequate interpretation of studies on blood glucose variability (GV) and diagnosis and monitoring of GDM, as well.
As a hypothesis, the rapid hormonal changes (38,39), which are established at the beginning of pregnancy, may play a relevant role in the greater variability of this assay in the period.
This work describes the current diagnosis of GDM. The knowledge of the magnitude of the variation for fasting glucose results is essential for the correct interpretation of this parameter and in the diagnosis of GDM. Changes in GDM frequency impact the workload and cost of the health system, medicalization, and stress of affected pregnant women (40-42).
In summary, fasting glucose is linked to the diagnosis of GDM and presents a wide variation, mostly derived from intra-individual biological variability (CVi). The effect of the small variation in FG is significantly greater at the beginning of pregnancy when compared to diagnosis with fasting glycemia in later stages.
Acknowledgments
Funding: This work was supported by CNPq, Araucaria Foundation and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-57/rc
Data Sharing Statement: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-57/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-57/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Federal University of Parana’s Ethics Committee (CAAE: 39460414.0.0000.0102) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16:7. [Crossref] [PubMed]
- Duncan JM. On puerperal diabetes. Trans Obstet Soc Lond 1982;24:256-85.
- Introduction: standards of medical care in diabetes-2020. Diabetes Care 2020;43:S1-S2. [Crossref] [PubMed]
- Diretrizes sociedade brasileira de diabetes 2019-2020 [Internet]. Clannad. 2019. Available online: http://www.saude.ba.gov.br/wp-content/uploads/2020/02/Diretrizes-Sociedade-Brasileira-de-Diabetes-2019-2020.pdf
- Coustan DR, Lowe LP, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome (hapo) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol 2010;202:654.e1-6. [Crossref] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676-82. [Crossref] [PubMed]
- O'SULLIVAN JB. MAHAN CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964;13:278-85. [PubMed]
- Houshmand A, Jensen DM, Mathiesen ER, et al. Evolution of diagnostic criteria for gestational diabetes mellitus. Acta Obstet Gynecol Scand 2013;92:739-45. [Crossref] [PubMed]
- Simmons D, Moses RG. Gestational diabetes mellitus: to screen or not to screen?: Is this really still a question? Diabetes Care 2013;36:2877-8. [Crossref] [PubMed]
- Miailhe G, Kayem G, Girard G, et al. Selective rather than universal screening for gestational diabetes mellitus? Eur J Obstet Gynecol Reprod Biol 2015;191:95-100. [Crossref] [PubMed]
- Corrado F, D'Anna R, Cannata ML, et al. Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis. Diabetes Metab 2012;38:458-61. [Crossref] [PubMed]
- Supak Smolcic V, Bilic-Zulle L, Fisic E. Validation of methods performance for routine biochemistry analytes at Cobas 6000 analyzer series module c501. Biochem Med (Zagreb) 2011;21:182-90. [Crossref] [PubMed]
- Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409-37. [Crossref] [PubMed]
- Braga F, Panteghini M. Generation of data on within-subject biological variation in laboratory medicine: An update. Crit Rev Clin Lab Sci 2016;53:313-25. [Crossref] [PubMed]
- Fraser CG. Reference change values. Clin Chem Lab Med 2011;50:807-12. [PubMed]
- Ricós C, Fernández-Calle P, Gonzalez-Lao E, et al. Critical appraisal and meta-analysis of biological variation studies on glycosylated albumin, glucose and HbA1c. Adv Lab Med 2020;1:20200029. [Crossref]
- Fraser CG. The necessity of achieving good laboratory performance. Diabet Med 1990;7:490-3. [Crossref] [PubMed]
- Ricós C, Perich C, Doménech M, et al. Variación biológica. Revisión desde una perspectiva práctica. Rev Lab Clín 2010;3:192-200. [Crossref]
- Hyltoft Petersen P, Klee GG. Influence of analytical bias and imprecision on the number of false positive results using guideline-driven medical decision limits. Clin Chim Acta 2014;430:1-8. [Crossref] [PubMed]
- Hyltoft Petersen P, Brandslund I, Jørgensen L, et al. Evaluation of systematic and random factors in measurements of fasting plasma glucose as the basis for analytical quality specifications in the diagnosis of diabetes. 3. Impact of the new WHO and ADA recommendations on diagnosis of diabetes mellitus. Scand J Clin Lab Invest 2001;61:191-204. [Crossref] [PubMed]
- Chan AY, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem 1989;35:315-7. [Crossref] [PubMed]
- Tran MT, Hoang K, Greaves RF. Practical application of biological variation and Sigma metrics quality models to evaluate 20 chemistry analytes on the Beckman Coulter AU680. Clin Biochem 2016;49:1259-66. [Crossref] [PubMed]
- Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes mellitus prevalence: Effect of the laboratory analytical variation. Diabetes Res Clin Pract 2015;109:493-9. [Crossref] [PubMed]
- Ricós C, Cava F, García-Lario JV, et al. The reference change value: a proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest 2004;64:175-84. [Crossref] [PubMed]
- Ko DH, Park HI, Hyun J, et al. Utility of Reference Change Values for Delta Check Limits. Am J Clin Pathol 2017;148:323-9. [Crossref] [PubMed]
- Braga F, Ferraro S, Ieva F, et al. A new robust statistical model for interpretation of differences in serial test results from an individual. Clin Chem Lab Med 2015;53:815-22. [Crossref] [PubMed]
- Carobene A. Reliability of biological variation data available in an online database: need for improvement. Clin Chem Lab Med 2015;53:871-7. [Crossref] [PubMed]
- Cooper G, DeJonge N, Ehrmeyer S, et al. Collective opinion paper on findings of the 2010 convocation of experts on laboratory quality. Clin Chem Lab Med 2011;49:793-802. [Crossref] [PubMed]
- Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med 2004;42:758-64. [Crossref] [PubMed]
- Ricós C, Iglesias N, García-Lario JV, et al. Within-subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem 2007;44:343-52. [Crossref] [PubMed]
- Nigam A, Sharma S, Varun N, et al. Comparative analysis of 2-week glycaemic profile of healthy versus mild gestational diabetic pregnant women using flash glucose monitoring system: an observational study. BJOG 2019;126:27-33. [Crossref] [PubMed]
- Trujillo J, Vigo A, Duncan BB, et al. Impact of the International Association of Diabetes and Pregnancy Study Groups criteria for gestational diabetes. Diabetes Res Clin Pract 2015;108:288-95. [Crossref] [PubMed]
- Yu W, Wu N, Li L, et al. A review of research progress on glycemic variability and gestational diabetes. Diabetes Metab Syndr Obes 2020;13:2729-41. [Crossref] [PubMed]
- Kim JA, Kim J, Roh E, et al. Association of fasting plasma glucose variability with gestational diabetes mellitus: a nationwide population-based cohort study. BMJ Open Diabetes Res Care 2020;8:e001084. [Crossref] [PubMed]
- Sesmilo G, Prats P, Garcia S, et al. First-trimester fasting glycemia as a predictor of gestational diabetes (GDM) and adverse pregnancy outcomes. Acta Diabetol 2020;57:697-703. [Crossref] [PubMed]
- Su JB, Wang XQ, Chen JF, et al. Glycemic variability in gestational diabetes mellitus and its association with β cell function. Endocrine 2013;43:370-5. [Crossref] [PubMed]
- Wei YM, Liu XY, Shou C, et al. Value of fasting plasma glucose to screen gestational diabetes mellitus before the 24th gestational week in women with different pre-pregnancy body mass index. Chin Med J (Engl) 2019;132:883-8. [Crossref] [PubMed]
- Catalano PM. Carbohydrate metabolism and gestational diabetes. Clin Obstet Gynecol 1994;37:25-38. [Crossref] [PubMed]
- Catalano PM. Trying to understand gestational diabetes. Diabet Med 2014;31:273-81. [Crossref] [PubMed]
- Benhalima K, Mathieu C, Damm P, et al. A proposal for the use of uniform diagnostic criteria for gestational diabetes in Europe: an opinion paper by the European Board & College of Obstetrics and Gynaecology (EBCOG). Diabetologia 2015;58:1422-9. [Crossref] [PubMed]
- Ryan EA. Diagnosing gestational diabetes. Diabetologia 2011;54:480-6. [Crossref] [PubMed]
- Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ 2014;348:g1567. [Crossref] [PubMed]
Cite this article as: Volanski W, Rego FGDM, Prado ALD, Signorini L, Picheth GF, Alves AC, Valdameri G, Picheth G. Small variations in fasting blood glucose have significant effects in diagnosis of gestational diabetes mellitus. J Lab Precis Med 2022;7:1.


