
Hemoglobin Shelby interfered with the leukocyte differential channel of the Mindray BC-6800Plus hematology analyzer: a case report
Introduction
Unstable hemoglobin (Hb) variants result from inherited mutations that affect globin genes. They decrease the solubility of the protein and cause intracellular precipitation. Approximately 150 Hb variants are unstable and cause chronic or episodic hemolysis. This may lead to hemolytic anemia of variable severity, depending on the extent of the molecular defect (1). Incidental detection of Hb variants is common in routine Hb A1c analyses. Abnormal patterns in the differential leukocytes (WBC) scattergrams can also indicate a variant Hb carrier, and unstable Hb forms have been identified using the WBC channel of the Sysmex analyzer (Sysmex Corporation, Kobe, Japan) (2-6).
The BC-6800 Plus hematology analyzer (Mindray Medical International Co., Shenzhen, China) uses technology and fluorescent reagents similar to those employed in Sysmex series analyzers.
WBC labelled with a fluorescence reagent are counted and classified in analytical channels using different detectors for scattered light (forward and side) and fluorescence signals. The intensity of the fluorescent signal (Y-axis in the WBC differential scattergram) reflects the amount of nucleic acids within the cell. The surfactant-induced lysis of the red blood cells (RBC) increases the permeability of WBC. Resistance to lysis of erythrocytes with a Hb variant could interfere with the normal functioning of the channel and alter the interaction of the dye with intracellular structures and nucleic acids, therefore resulting in a characteristic abnormal pattern. We present the following case in accordance with the CARE reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-56/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
We present a case of an incidental finding of a Hb variant. An asymptomatic 47-year-old woman born in Bolivia was referred to our center, Hospital Galdakao Usansolo, for a routine health check.
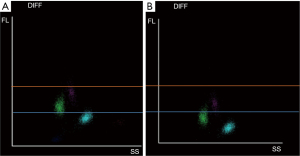
Analytical data are shown in Table 1. The complete blood count (CBC) was normal. Reticulocytosis was detected, but the WBC scatterplot did not show decreased signals (Figure 1). Target RBCs were found on the peripheral blood smear, but there were no other alterations in blood cell morphology. This is not a typical finding for hemoglobinopathies. Rather, target RBCs can usually be detected in patients who had liver disease or a post splenectomy.
Table 1
| Parameters | Patient results | Reference interval |
|---|---|---|
| Erythrocytes | 4.86×1012/L | (3.80–5.40)×1012/L |
| Hb | 120 g/L | 120–155 g/L |
| Hematocrit | 39.8% | 35.5–45.0% |
| MCV | 88.1 fL | 80.0–98.0 fL |
| MCH | 28.4 pg | 27.0–32.0 pg |
| MCHC | 322 g/L | 330–360 g/L |
| RDW | 15.1% | 11.5–16.0% |
| Platelets | 294×109/L | (150–450)×109/L |
| Leukocytes | 8.40×109/L | (4.0–11.0)×109/L |
| Neutrophils | 5.06×109/L (60.2%) | (1.5–7.5)×109/L |
| Lymphocytes | 2.70×109/L (32.1%) | (1.3–3.0)×109/L |
| Monocytes | 0.46×109/L (5.5%) | (0.1–0.8)×109/L |
| Eosinophils | 0.17×109/L (2.0%) | (0.02–0.4)×109/L |
| Basophils | 0.02×109/L (0.2%) | (0.0–0.2)×109/L |
| Reticulocytes | 314×109/L | (25–75)×109/L |
| Glucose | 4.94 mmol/L | 4.22–5.55 mmol/L |
| Creatinine | 63.0 µmol/L | 53.0–106.0 µmol/L |
| Ferritin | 469 pmol/L | 67.4–674.1 pmol/L |
| Haptoglobin | 4.3 µmol/L | 3.5–20.0 µmol/L |
| Total bilirubin | 11.9 µmol/L | 1.71–18.8 µmol/L |
| LDH | 159 U/L | 135–250 U/L |
Hb, hemoglobin; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; RDW, red cell distribution width; LDH, lactate dehydrogenase.
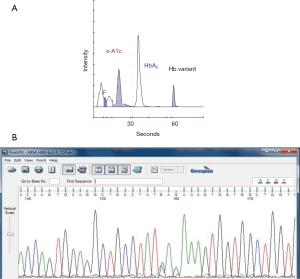
A chromatograph obtained on an ADAMS A1c, HA-8180V high performance liquid chromatography (HPLC) analyzer (Arkray Inc., Kyoto, Japan) showed a peak of 13% for a Hb variant with the same retention time as Hb C. DNA sequencing was performed to pinpoint the exact nature of the alteration. A missense mutation of CAG to AAG at codon 131 in one of the β-globin alleles was detected, which is a variant called Hb Shelby (7) (Figure 2).
Discussion
The variant has been detected in several African American families. Some of them had the Hb trait, while others were double heterozygotes who carried Hb Shelby in combination with other hemoglobinopathies, such as α- and β-thalassemia HbS or HbC (8). The Hb Shelby variant affects the α–β bridging of the Hb molecule, which results in mildly instability and affects its solubility (8). This is consistent with the target cells observed in the peripheral blood smear in the case described here. Target cells form when peripheral blood smears air dry and have a large surface to volume ratio of RBCs (9). The increased retention time of Hb Shelby as shown on an ion-exchange HPLC is due to the substitution of lysine (a basic amino acid) for glutamine (a neutral amino acid), which renders a molecule with an additional positive charge (8,9).
In our case, the hemoglobinopathy was not clinically significant, in agreement with the previous reports. The Hb Shelby and the compound heterozygotes followed a benign clinical course, and only some individuals displayed mild anemia (8,9). Despite the instability of the variant, our patient had relatively normal laboratory results; she only had an increase in reticulocyte count, which compensated for the mild hemolysis.
To the best of our knowledge, this is the first case of Hb Shelby being detected using a Mindray analyzer. Our observations confirm an earlier report (10); the scattergram displayed similarly reduced fluorescence signals in Hb Johnstown carriers. This is a high-oxygen-affinity variant; the reduced release of oxygen leads to tissue hypoxia with a compensatory increase in erythropoiesis and subsequent increases in secondary erythrocytosis and polycythemia. The abnormality flags for those variant carriers reflect the pathological Hb and red cell index values. However, in the case of unstable Hb variants, mild hemolytic anemia can be the only clinical evidence. The minimal changes in CBC are difficult to observe and often mean that the condition is undetected in most cases.
Cell population data (CPD) are the numerical coordinates of the differential WBC clusters on the WBC scatterplot. The CPD values, shown on the Y-axis, are distinctly reduced in unstable Hb carriers. This observation could be useful for optimizing the detection of RBC lysis resistance because their altered values could be a trigger for detection. Identifying the pattern of an abnormal WBC scattergram could help select samples for further examination of a potential Hb variant carrier. In conjunction with clinical evidence of hemolytic anemia, such flagging should trigger a search for an unstable Hb variant. Since these diagnoses can have important implications, the family members must also be examined as there might be a risk of severe forms of hemoglobinopathy in future generations. The CBC could be a cost-effective tool for detecting such patients. Our case shows that a WBC scattergram with an abnormally low fluorescent signal, obtained on the Mindray BC6800 Plus analyzer, indicates the presence of a Hb variant and warrants further investigation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-56/rc
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-56/coif). EU serves as an unpaid editorial board member of The Journal of Laboratory and Precision Medicine from December 2021 to November 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rizzuto V, Koopmann TT, Blanco-Álvarez A, et al. Usefulness of NGS for Diagnosis of Dominant Beta-Thalassemia and Unstable Hemoglobinopathies in Five Clinical Cases. Front Physiol 2021;12:628236. [Crossref] [PubMed]
- Rosetti M, Poletti G, Sensi A, et al. A rare case of Hemoglobin Leiden interfering with the DIFF channel of Sysmex XE-2100. Scand J Clin Lab Invest 2015;75:436-7. [Crossref] [PubMed]
- Rosetti M, Poletti G, Ivaldi G, et al. Serendipitous detection of Hemoglobin G-Ferrara variant with Sysmex DIFF channel. Clin Biochem 2016;49:192-3. [Crossref] [PubMed]
- Teixeira C, Pina D, Freitas MI. Automated detection of unstable hemoglobin variants by Sysmex XE-Series analyzers. Clin Chem Lab Med 2017;55:e243-6. [Crossref] [PubMed]
- Jongbloed W, van Twillert G, Schoorl M, et al. Unstable haemoglobin variant Hb Leiden is detected on Sysmex XN-Series analysers. Clin Chem Lab Med 2018;56:e249-50. [Crossref] [PubMed]
- Park S, Jeong TD, Hong KS, et al. A Clue to Discovering Unstable Hemoglobin Variants via Abnormal WBC Differential Scattergrams Using the Sysmex Automated Hematology Analyzer. Lab Med Online 2019;9:84-7. [Crossref]
- Moo-Penn WF, Johnson MH, McGuffey JE, et al. Hemoglobin Shelby [beta 131(H9) Gln—Lys] a correction to the structure of hemoglobin Deaconess and hemoglobin Leslie. Hemoglobin 1984;8:583-93. [Crossref] [PubMed]
- Wagner SC, Pereira C, Castro SM. Association of Hb Shelby with Hb S in the south of Brazil. Haematologica 2006;91:1141-2. [PubMed]
- Scuderi RT, Griffin TL, Mehta SP, et al. Interference with hemoglobin A(1C) determination by the hemoglobin variant Shelby. Am J Clin Pathol 2007;128:440-4. [Crossref] [PubMed]
- Urrechaga E, Fernández M, Orbe RD. Hb Johnstown is detected on Mindray BC 6800 Plus analyzer. Clin Chem Lab Med 2021;59:e386-8. [Crossref] [PubMed]
Cite this article as: Urrechaga E, Merino M, Fernández M, Aguirrezabal J, del Orbe R. Hemoglobin Shelby interfered with the leukocyte differential channel of the Mindray BC-6800Plus hematology analyzer: a case report. J Lab Precis Med 2022;7:14.


