
Positive findings in repeated serum protein electrophoresis tests after an initial negative result in patients without prior history of plasma cell disorders: a pilot retrospective database study
Introduction
Inappropriate pathology test requests may result in healthcare resource wastages and cause patient harm (1). There have been efforts to promote evidence-based laboratory test requesting practice that creates value for patients and the healthcare system (2). Examples of such efforts include the Choosing Wisely campaign led by the American Board of Internal Medicine Foundation (https://www.choosingwisely.org/) and the national minimum retesting intervals recommended by the Royal College of Pathologists, The Association for Clinical Biochemistry and Laboratory Medicine and Institute of Biomedical Science (1,3). Serum protein electrophoresis is a pathology test that detects monoclonal immunoglobulins (4). It is primarily used to screen for plasma cell disorders, in conjunction with serum free light chain measurement (4). At present, there is limited evidence on the diagnostic performance of repeat serum protein electrophoresis after an initial negative result. In this retrospective observational study, we reported the utility of repeat serum protein electrophoresis testing for new findings of monoclonal proteins at a large tertiary health network. The following article is presented in accordance with the STROBE reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-67/rc).
Methods
Northern Pathology Victoria is a public pathology provider for Northern Health and the Northern community in Epping, Australia. It is accredited by The National Association of Testing Authorities. All serum protein electrophoresis performed for adult patients (≥18 years old) between 8 January 2019 and 17 November 2020 at Northern Pathology Victoria were retrospectively reviewed. Serum protein electrophoresis was performed using the high-resolution agarose gel electrophoresis and immunofixation electrophoresis (Hydrasys, Sebia, France) on a semi-automated platform. Any observed monoclonal band is quantitated by densitometry. The agarose gel electrophoresis system has a lower reporting limit of 1 g/L for serum monoclonal protein quantitation as recommended by the Working Party on Standardised Reporting of Protein Electrophoresis of the Australasian Association for Clinical Biochemists (5). Agarose gels were interpreted by two trained laboratory scientists. All laboratory procedures were performed according to manufacturer’s instructions with acceptable quality control and proficiency testing performances recorded during the study period.
For each serum protein electrophoresis request, the date of collection, test result, patient demographics and clinical indication were extracted from the laboratory information system and clinical records. For patients whose serum protein electrophoresis findings changed from negative to positive monoclonal band(s), the medical records were reviewed to determine whether there was any new diagnosis of plasma cell disorders. The clinical indications were broadly categorised into: abnormal serum globulin and/or total immunoglobulins, proteinuria and/or albuminuria and/or haematuria, abnormal peripheral blood smear, anaemia and/or leukopaenia and/or thrombocytopaenia, chronic kidney disease and/or acute kidney injury, anaemia with chronic kidney disease and/or acute kidney injury, hypercalcaemia, previously abnormal serum protein electrophoresis and/or serum free light chains, osteoporosis and/or osteolytic bone lesions, skin changes, peripheral neuropathy, malignancy and/or autoimmune conditions.
For patients whose serum protein electrophoresis findings changed from negative to positive monoclonal band(s), the medical records were reviewed to determine whether there was any new diagnosis of plasma cell disorders. In this study, repeat testing was defined as any serum protein electrophoresis test done more than once in a patient within the two-year study period. Patients with missing clinical information or had known plasma cell and/or lymphoproliferative disorders were excluded from further analysis. All clinical data were de-identified prior to analysis. The extracted laboratory data were summarised using descriptive statistics. The clinical information for patients who had positive repeat serum protein electrophoresis testing during the study period was summarised and reported as clinical vignettes. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the Northern Health Human Research Ethics Committee (No. ALR 33.2021) and individual consent for this retrospective analysis was waived.
Results
In the 23-month study period, there were 4,101 serum protein electrophoresis tests performed in 2,730 patients (median age, 69 years; range, 18–98 years). Repeat testing was performed on 566 (20.7%) of 2,730 patients, accounting for 1,371 (33.4%) of the total tests requested. After excluding patients with a history of plasma cell and/or lymphoproliferative disorders, a total of 160 of the 2,730 patients (5.9%) had two or more serum protein electrophoresis requests. Approximately half (51%) of the repeat tests were requested by a clinician of the same clinical specialty as the initial requestor. For 56% of the repeat requests the clinical indication for serum protein electrophoresis was broadly the same as for the initial request.
Serum protein electrophoresis was repeated once in 144 (90.0%) subjects, twice in 12 (7.5%) individuals and three times in four (2.5%) patients. Between the initial (negative) and repeat requests for serum protein electrophoresis, about two-thirds of the repeats were performed within the first six months of the initial test (Figure 1).
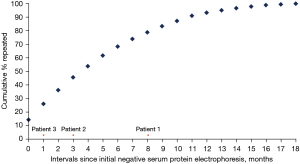
Only three (1.9%) out of the 160 patients with an initial negative test had positive results on subsequent requests. All three patients had low level of monoclonal protein band (≤2 g/L), which is just above the lower limit of serum monoclonal protein quantitation (1 g/L) for the protein electrophoresis method. For two patients, although the serum free light chains co-requested with the initial serum protein electrophoresis showed abnormal measurements, the serum kappa-to-lambda free light chain ratio was normal relative to glomerular filtration rate-specific reference interval. A follow-up review of the clinical notes of the three patients at least 16 months after the second, positive serum protein electrophoresis tests revealed that none of them were diagnosed with plasma cell disorder, including monoclonal gammopathy of undetermined significance (Table 1).
Table 1
| Patient age and gender | Main indication for SPE request | SPE result | Clinical information |
|---|---|---|---|
| Patient 1 (57-year-old female) | First request: anaemia | First request (May 2019): monoclonal protein was not detected | Past history also included chronic kidney disease, anaemia, ulcerative colitis with short bowel syndrome |
| Second request: work-up for osteoporosis | Second request (January 2020): IgG lambda monoclonal protein, 2 g/L | Laboratory measures associated with first request: | |
| Third request (May 2020): IgG lambda monoclonal protein, 1 g/L | Monoclonal protein was not detected on urine protein electrophoresis; serum free light chain measurements were within reference interval | ||
| Fourth request (March 2021): IgG lambda monoclonal protein, 2 g/L | Bone marrow examination was not performed following the second SPE findings | ||
| As of 1 November 2021, patient was not diagnosed with plasma cell disorder and there was no further serum protein electrophoresis performed for this patient | |||
| Patient 2 (59-year-old male) | First request: chronic kidney disease | First request (March 2020): monoclonal protein was not detected | Past history also included Hashimoto’s thyroiditis, hypertension |
| Second request: peripheral neuropathy | Second request (June 2020): small IgG kappa band, approximately 1 g/L, on background of polyclonal and/or oligoclonal pattern, with uncertain clinical significance (may reflect an inflammatory process) | Other relevant laboratory measures associated with first request: | |
| (I) eGFR: 49 mL/min/1.73 m2 (reference, ≥60 mL/min/1.73 m2) | |||
| (II) Serum free light chains: | |||
| • Free kappa: 38.0 mg/L (reference, 3.3–19.3 mg/L) | |||
| • Free lambda: 22.7 mg/L (reference, 5.7–26.3 mg/L) | |||
| • Kappa-to-lambda ratio: 1.67 (*reference, 0.26–1.65) | |||
| (III) Monoclonal protein was not detected on urine protein electrophoresis | |||
| As of 1 November 2021, patient was not diagnosed with plasma cell disorder and there was no further serum protein electrophoresis performed for this patient | |||
| Patient 3 (82-year-old male) | First request: gait difficulty, peripheral neuropathy | First request (February 2019): monoclonal protein was not detected | Past history also included ischaemic heart disease, dilated cardiomyopathy, strokes, colorectal cancer, arthritis |
| Second request: recurrent falls | Second request (March 2019): small IgA lambda band only detectable by immunofixation | Serum free light chains: | |
| • Free kappa: 21.3 mg/L (reference, 3.3–19.3 mg/L) | |||
| • Free lambda: 38.6 mg/L (reference, 5.7–26.3 mg/L) | |||
| • Kappa-to-lambda ratio: 0.55 (reference, 0.26–1.65) | |||
| As of 1 November 2021, patient was not diagnosed with plasma cell disorder and there was no further serum protein electrophoresis performed for this patient |
*, for serum free light chains, a ‘renal’ reference interval for kappa-to-lambda ratio of 0.37–3.10 may be more appropriate for a patient with reduced glomerular filtration rate. SPE, serum protein electrophoresis; Ig, immunoglobulin; eGFR, estimated glomerular filtration rate.
Discussion
Monoclonal gammopathy of undetermined significance is a precursor to multiple myeloma (6). In a study of Olmsted County residents aged 50 years or older, monoclonal gammopathy of undetermined significance was identified in 3.2% of 21,463 subjects (6). The risk of progression to multiple myeloma, Waldenstrom’s macroglobulinaemia, light chain (AL) amyloidosis or a lymphoproliferative disorder is reportedly approximately 1% per year (6). Sigurdardottir and colleagues reported that patients with multiple myeloma with prior diagnosis of monoclonal gammopathy of undetermined significance had better survival, suggesting that earlier treatment of multiple myeloma leads to better survival (7). Hence, there is a clinical desire for early detection by screening asymptomatic patients (8).
A consequence of increased screening is the increased number of patients that must be followed up particularly for those with negative results. However, the evidence for repeat serum protein electrophoresis testing following a negative initial result is lacking. The Royal College of Pathologists suggested annual serum protein electrophoresis and quantitation by densitometry without the need for further immunofixation for patients without features of plasma cell disorders and a monoclonal protein concentration of <15 g/L (3). No specific recommendation is made for patients without prior history of plasma cell disorder and have a negative serum protein electrophoresis result.
In this pilot study, only three of the 160 patients without prior history of plasma cell and/or lymphoproliferative disorders had positive repeat serum protein electrophoresis after an initial negative result. The precise conversion rate cannot be determined in this study owing to the small sample size and a lack of standardised retesting intervals. A larger prospective cohort with protocolised repeat testing and a longer follow-up period is required to document the clinical progression of this group of patients.
Taken together, the findings of this pilot study provided additional supporting evidence to the one-year retesting interval for serum protein electrophoresis for patients without features of plasma cell disorders and with a monoclonal protein concentration of <15 g/L, as recommended by the Royal College of Pathologists (UK) (3).
There are several limitations in this study that are important to consider when interpreting the findings. The data described in this study were obtained from a single urban health pathology laboratory in Australia and may limit its generalisability to other regions that may have significantly different patient demographics or clinical practice. The relatively short study period and the retrospective, cross-sectional observational study design also limits the strength of evidence. Cost-effectiveness assessment may provide additional evidence to inform the optimal follow-up strategy of a negative serum protein electrophoresis finding.
Acknowledgments
The abstract of this article has been published in Pathology.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-67/rc
Data Sharing Statement: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-67/dss
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-67/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-67/coif). TPL serves as an unpaid Associate Editor-in-Chief of Journal of Laboratory and Precision Medicine from July 2021 to December 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the Northern Health Human Research Ethics Committee (No. ALR 33.2021) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Levinson W, Kallewaard M, Bhatia RS, et al. 'Choosing Wisely': a growing international campaign. BMJ Qual Saf 2015;24:167-74. [Crossref] [PubMed]
- Xu S, Hom J, Balasubramanian S, et al. Prevalence and Predictability of Low-Yield Inpatient Laboratory Diagnostic Tests. JAMA Netw Open 2019;2:e1910967. [Crossref] [PubMed]
- The Royal College of Pathologists. The Association for Clinical Biochemistry and Laboratory Medicine, Institute of Biomedical Science. National Minimum Retesting Intervals in Pathology. Accessed June 24, 2021. Available online: https://www.rcpath.org/discover-pathology/news/national-minimum-retesting-intervals.html/
- McTaggart MP, Kearney EM. Evidence-based use of serum protein electrophoresis in laboratory medicine. Clin Chem Lab Med 2013;51:e113-5. [Crossref] [PubMed]
- Tate J, Caldwell G, Daly J, et al. Recommendations for standardized reporting of protein electrophoresis in Australia and New Zealand. Ann Clin Biochem 2012;49:242-56. [Crossref] [PubMed]
- Therneau TM, Kyle RA, Melton LJ 3rd, et al. Incidence of monoclonal gammopathy of undetermined significance and estimation of duration before first clinical recognition. Mayo Clin Proc 2012;87:1071-9. [Crossref] [PubMed]
- Sigurdardottir EE, Turesson I, Lund SH, et al. The Role of Diagnosis and Clinical Follow-up of Monoclonal Gammopathy of Undetermined Significance on Survival in Multiple Myeloma. JAMA Oncol 2015;1:168-74. [Crossref] [PubMed]
- Graziani MS, Merlini G. Recommendations for appropriate serum electrophoresis requests: the Italian approach. Clin Chem Lab Med 2013;51:e117-8. [Crossref] [PubMed]
Cite this article as: Choy KW, Abcede JG, Chong YP, Loh TP. Positive findings in repeated serum protein electrophoresis tests after an initial negative result in patients without prior history of plasma cell disorders: a pilot retrospective database study. J Lab Precis Med 2022;7:9.


