
Association of age, allergic rhinitis, and regular food intake with urinary tetranor-PGD metabolite levels
Serum concentrations of prostaglandin D2 (PGD2), a biologically active substance produced by mast cells tend to increase during immediate allergic reactions. PGD2 is metabolized and excreted in urine as tetranor-PGD metabolite (PGDM). We previously reported urinary PGDM as a sensitive biomarker for food allergic reactions in children (1-3), although its specificity remains unknown. Importantly, we also reported that urinary PGDM levels did not change in atopic dermatitis, another model of mast cell-related allergic condition (4). Here, we determined the changes in urinary PGDM levels in allergic rhinitis (AR), a mucosal inflammatory condition caused by inhaled allergens such as pollen and is driven by activated immune cells including mast cells (5,6). Further, the reference range of PGDM is not validated and food allergy can affect adults as well. Therefore, we also examined the effect of ageing and regular food intake on urinary PGDM levels.
To evaluate the relationship between urinary PGDM levels and pollen-related AR, we recruited adults aged ≥15 years with cedar pollen allergy in National Center for Child Health and Development, in Tokyo, Japan between December 2020 and January 2021. Participants were excluded if they had any other uncontrolled allergic diseases. Cedar pollen allergy was defined based on self-reporting and study physicians’ assessment. Spot urine samples were collected once before (end of January) and once during (mid-February to April) the cedar pollination season (7). We defined total nasal symptom and medication score (TNSMS) of 0 as asymptomatic and TNSMS score of ≥1 as symptomatic, as previously described (7).
To evaluate the relationship of urinary PGDM with age and intake of regular meals not evoking allergy, we examined healthy participants aged >1 year recruited between May 2020 and December 2020 in National Center for Child Health and Development in Tokyo, Japan. Participants were eligible if they had not had atopic dermatitis, asthma and allergic rhinitis before. We classified participants into three groups according to age: preschooler, aged ≤5 years; school-aged, aged 6–14 years; adolescent and adult, aged ≥15 years. We collected urine samples before and four hours after regular meals. We measured urinary PGDM levels using liquid chromatography-tandem mass spectrometry and expressed the obtained values as ng/mg creatinine (ng/mg Cre) after normalizing to creatinine levels. All statistical analyses were performed with EZR version 1.30 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). The study was approved by the ethical committee of the National Center for Child Health and Development and the University of Tokyo (Registration No. 2127) and all participants or their legal representatives provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Eleven participants with AR were recruited. The age range of the participants was 15 to 59 years, with a median age of 31.0 years. For all participants, TNSMS =0 before the pollination season and the median (IQR) TNSMS was 7.0 (2–14) during the pollination season. The baseline median [interquartile range (IQR)] PGDM level was 1.48 (0.89–1.69) ng/mg Cre (Table S1 and Figure S1). The median (IQR) urinary PGDM levels were not significantly different between the samples collected before and during the pollination season [1.48 (0.91–1.67) and 1.13 (0.66–1.35) ng/mg Cre, respectively; P=0.206, Wilcoxon’s signed-rank test; Figure 1A].
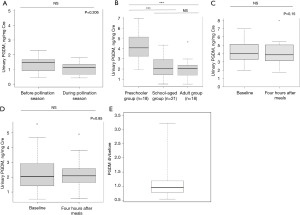
Fifty-seven healthy volunteers were included in the analysis of changes in urinary PGDM levels according to age and regular food intake. Previously, we have reported that when allergic symptoms are induced in food allergy patients, urinary PGDM levels peak 4 hours after ingestion of the causative food. For this reason, urine samples were also collected 4 hours after food intake in this study. The baseline median (IQR) PGDM level was 2.44 (1.74–3.71) ng/mg Cre (Figure S2). The urinary PGDM levels were higher in the preschool group [median (IQR): 4.03 (3.36–5.06) ng/mg Cre] compared with the school-age and adult groups [2.37 (1.56–3.10) and 1.97 (1.29–2.35) ng/mg Cre, respectively; P<0.001, Kruskal-Wallis test; Figure 1B], whereas no significant difference was noted between the school-age and adult groups. Regular meal intake did not alter PGDM level regardless of age [median (IQR): 4.03 (3.36–5.06) vs. 3.84 (3.14–4.95) ng/mg Cre; P=0.85 Wilcoxon signed rank test; Figure 1C-1E].
In this study, urine samples were collected as spot urine and subjected to mass spectrometry without any special process other than the addition of reagents, thus requiring only a very small amount. The noninvasive nature of the urine collection makes this method useful for evaluating patients with allergic diseases, including children. In addition, other allergic mediators, such as histamine and tryptase, are unstable substances and require special considerations in specimen collection time and storage conditions. In comparison, PGDM is a stable substance and easy to handle. In the future, we are considering developing a dedicated testing device to simplify the analysis.
Together with our previous finding in atopic dermatitis, the lack of a significant difference in urinary PGDM levels in the collected samples before and during the pollination season from participants with AR in the current study suggest that PGDM might be specific to food allergy and might distinguish between food allergic reaction from mast cell-induced local allergy. Food allergy is dependent on alimentary tract absorption and systemic response, whereas atopic dermatitis and AR are local allergic reactions that occur in skin and nasal mucosa, respectively. This difference in the extent of mast cell activation might at least partially explain the specificity of PGDM in food allergy, which should be evaluated in future studies.
We also demonstrated that baseline urinary PGDM levels declined with age. The baseline urinary PGDM levels were higher in participants younger than five years of age compared to older participants. We standardized PGDM to creatinine, which is a byproduct of muscle metabolism that is continually excreted primarily through glomerular filtration and partly secreted by renal peritubular capillaries (8). In healthy individuals, urinary creatinine concentrations correlate with anthropometric measurements, such as body mass and body mass index (9), which may account for higher urinary PGDM levels adjusted to creatinine in preschoolers with less muscle mass and lower serum creatinine levels. Therefore, using cystatin- C, which is not dependent on muscle mass, to standardize urinary PGDM levels might be useful in children. In our previous studies, we normalized PGDM values with creatinine as well. To be consistent with these studies, we also normalized PGDM values with creatinine in this study. Furthermore, in the protocol for this study, approved by the IRC, the creatinine measurement was allowed, but the measurement of cystatin C was not. We would like to update our protocol to allow the measurement of cystatin C in future studies. Large-scale surveys are warranted to determine age-dependent standard reference values as well as the cutoff values for food allergy. Finally, we found that meal intake did not impact urinary PGDM levels, providing further support that urinary PGDM is specific to food allergic reaction.
We could not evaluate urinary PGDM levels in infants, which was a limitation of our study. However, Ito et al. have recently developed a method to measure PGDM in urine collected via diapers (10), which we will utilize to validate our findings in future studies including infants. We also recognize the small number of participants as a limitation of the present study, which was designed as an exploratory investigation.
In conclusion, urinary PGDM levels were not affected by meal intake and AR symptoms. In addition, urinary PGDM levels differed among specific age groups.
Acknowledgments
We thank all our colleagues at the medical center and university who were involved in collecting urine samples and measuring the urinary PGDM levels.
Funding: This study was supported by a Grant-in-Aid for Scientific Research A from the Japan Society for the Promotion of Science (Grant Nos. 17921666, 20H05678, 18K14603) and Medical Research and Development Programs Focused on Technology Transfers: Development of Advanced Measurement and Analysis Systems from Japan Agency for Medical Research and Development, AMED (Grant No. 17935758).
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-46/coif). TS serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from September 2021 to August 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethical committee of the National Center for Child Health and Development and the University of Tokyo (Registration No. 2127) and all participants or their legal representatives provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maeda S, Nakamura T, Harada H, et al. Prostaglandin D2 metabolite in urine is an index of food allergy. Sci Rep 2017;7:17687. [Crossref] [PubMed]
- Inagaki S, Maeda S, Narita M, et al. Urinary PGDM, a prostaglandin D2 metabolite, is a novel biomarker for objectively detecting allergic reactions of food allergy. J Allergy Clin Immunol 2018;142:1634-1636.e10. [Crossref] [PubMed]
- Inuzuka Y, Yamamoto-Hanada K, Nakamura T, et al. Detection of allergic reactions during oral food challenge using noninvasive urinary prostaglandin D2 metabolites. Clin Exp Allergy 2022;52:176-9. [Crossref] [PubMed]
- Inagaki S, Nakamura T, Hamasaki Y, et al. Prostaglandin D2 metabolite is not a useful clinical indicator for assessing atopic dermatitis. Clin Exp Dermatol 2021;46:130-4. [Crossref] [PubMed]
- Meng Y, Wang C, Zhang L. Recent developments and highlights in allergic rhinitis. Allergy 2019;74:2320-8. [Crossref] [PubMed]
- Steelant B, Seys SF, Van Gerven L, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol 2018;141:951-963.e8. [Crossref] [PubMed]
- Yonekura S, Gotoh M, Kaneko S, et al. Treatment duration-dependent efficacy of Japanese cedar pollen sublingual immunotherapy: Evaluation of a phase II/III trial over three pollen dispersal seasons. Allergol Int 2019;68:494-505. [Crossref] [PubMed]
- Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005;113:192-200. [Crossref] [PubMed]
- Gerchman F, Tong J, Utzschneider KM, et al. Body mass index is associated with increased creatinine clearance by a mechanism independent of body fat distribution. J Clin Endocrinol Metab 2009;94:3781-8. [Crossref] [PubMed]
- Ito N, Nakamura T, Sakamoto N, et al. Extraction and measurement of urinary tetranor-PGDM in disposable diapers. J Pharmacol Sci 2021;147:208-10. [Crossref] [PubMed]
Cite this article as: Shimada M, Yamamoto-Hanada K, Inuzuka Y, Kabashima S, Nakamura T, Shimosawa T, Murata T, Ohya Y. Association of age, allergic rhinitis, and regular food intake with urinary tetranor-PGD metabolite levels. J Lab Precis Med 2022;7:36.


