
Nationwide analysis of COVID-19 death rate throughout the pandemic in Italy
Highlight box
Key findings
• Although our findings underpin a reassuring epidemiological scenario, with death rate for coronavirus disease 2019 (COVID-19) currently lower than that for common flu, active surveillance should be maintaining for preventing further surge of aggressiveness of SARS-CoV-2.
What is known and what is new?
• Mortality for COVID-19 has gradually declined over time, due to the combined effect of virus mitigation and herd (natural or vaccine-elicited) immunity.
• In this work, we estimated that the current death rate of COVID-19 is around 0.2%, thus lower than that of common flu (i.e., around 0.3%).
What is the implication, and what should change now?
• Some restrictive measures could be relieved, provided that active epidemiologic surveillance is maintained to intercept future hazards.
Introduction
Coronavirus disease 2019 (COVID-19), a respiratory infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), has been first identified in the Chinese town of Wuhan in November 2019, and has since been declared pandemic by the World Health Organization (WHO) in March 2020 (1), becoming the seven most deadly pandemics throughout the reported human history (2). The clinical course of this infectious disease has changed substantially over time, due to a number of biological and clinical aspects that have contributed to attenuate the impact of the virus on human health (3).
It is now undeniable that the major knowledge that we have progressively garnered on the pathogenesis of COVID-19, which might potentially become a life-threatening systemic disorder (i.e., thus causing injuries to a vast array of human organs and tissues) rather than remaining an isolated pulmonary disease (4), has enabled to considerably ameliorate the managed care of patients with SARS-CoV-2 infection, by adopting an ample armamentarium of conventional, innovative and even revolutionary medications, encompassing antiviral agents (i.e., anti-SARS-CoV-2 monoclonal antibodies, convalescent plasma, intravenous immune globulins, virucidal agents), anti-inflammatory and immunosuppressive drugs, anticoagulants (e.g., heparin and direct oral anticoagulants) and supportive treatments (i.e., supplemental oxygen, prone position), among others (5).
Three other aspects that have enormously contributed to mitigate the clinical and social burden of SARS-CoV-2 infection encompass the organization of widespread COVID-19 vaccination campaigns in several countries, acquisition of natural (herd) immunity and accumulation of less lethal mutations within the single-stranded RNA viral genome of SARS-CoV-2 (6). As concerns the former aspect, a huge number of COVID-19 vaccines (mRNA-based, protein-based, adenoviral, attenuated) have now become commercially available or are nearly ready to be approved (7), with some of these that have already progressed towards their “second” generation, being constructed with sequences of the original prototype SARS-CoV-2 virus combined with those of the recent Omicron BA.4/5 sublineages (i.e., the so-called “bivalent vaccines”) (8). Although the number of human lives that COVID-19 vaccines have helped to save throughout the pandemic cannot be definitely established, and will probably remain incalculable forever, it has been estimated that over 60% of SARS-CoV-2 related deaths could have been saved during the first year of COVID-19 vaccination, worldwide (9). A landscape of genomic mutations have also gradually accumulated over time in the RNA sequence of SARS-CoV-2, especially in that of the spike protein and its receptor biding domain (RBD) (10). Reliable evidence has now been provided that most of these genetic polymorphisms have rendered the original virus more fit to infect its human host, concomitantly expressing a globally less pathogenetic potential on human cells (11,12). It is hence not surprising that widespread perception is gaining momentum that the clinical severity of the disease caused by the currently predominant SARS-CoV-2 Omicron sublineages may be comparable to that caused by common flu (13).
Therefore, since no definitive epidemiological evidence has been published about the mutated SARS-CoV-2 lethality due to variant of concerns (VoCs) predominance and COVID-19 vaccination in relation to Influenza virus fatality to the best of our knowledge, we provide here an updated analysis of the COVID-19 death rate throughout the pandemic in Italy.
Methods
The number of new SARS-CoV-2 infections and COVID-19 related deaths was collected from official data reported by the WHO in its Coronavirus (COVID-19) Dashboard between February 2020 (when the first indigenous case was diagnosed in Italy) and present time (i.e., up to November 11, 2022), after setting the geographical location to Italy (14). Data on SARS-CoV-2 VoCs predominance and roll out of COVID-19 vaccination in Italy (encompassing both the primary cycle and booster doses administration) were accessed from the official website of the Italian National Institute of Health (15). As concerns influenza infections and mortality in the country, information was retrieved from the Italian National Institute of Health report published by Rosano et al. in 2019 (16), relative to the influenza seasons 2013/14 to 2016/17. Raw data were imported into a Microsoft Excel worksheet (Microsoft, Redmond, WA, United States), were they were graphically plotted, with calculation of the death rate for both COVID-19 and common flu. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This analysis was based on electronic searches in open and publicly available repositories, so that no informed consent or approvals from ethical committee were necessary.
Results
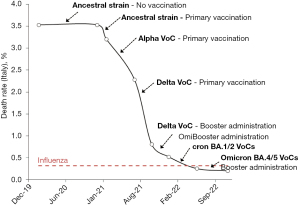
The progressive genetic evolution of SARS-CoV-2, with appearance of the four leading VoCs that have become predominant in Italy since the beginning of the COVID-19 pandemic is summarized in Table 1, compounded by the progression of COVID-19 primary vaccination and booster dose administration. Thus, not less than seven different periods could be identified by combining the surge of SARS-CoV-2 VoCs and the progression of the COVID-19 vaccination. The number of new diagnosed COVID-19 cases, COVID-19 attributable deaths and the resulting death rate is also reported in Table 1. Although a dramatic increase of new COVID-19 cases can be noted during the Omicron surge, as a result of the growth advantage of these compared to former SARS-CoV-2 VoCs in Italy and in most other countries (17), the COVID-19 death rate has instead exhibited a dramatic decline since the diagnosis of the first Italian case, in February 2020. Specifically, the mortality rate has declined from 3.53% during the ancestral SARS-CoV-2 strain predominance to 0.26% and 0.21% after surge of Omicron sublineages BA.1/2 and BA.4/5, respectively, when COVID-19 vaccine coverage (primary cycle) has also been extended to 90.2% of the national population aged 12 years of older, with 84.5% of these also receiving at least one COVID-19 vaccine booster dose (18).
Table 1
| SARS-CoV-2 VoC | Vaccination | Period | Duration (days) | COVID-19 | ||||
|---|---|---|---|---|---|---|---|---|
| Total cases | Daily cases | Attributable deaths | Daily attributable deaths | Death rate | ||||
| Ancestral | No | February 2020 to December 2020 | 314 | 2,083,698 | 6,636 | 73,604 | 234 | 3.53% |
| Ancestral | Primary cycle | January 2020 to January 2021 | 50 | 458,085 | 9,162 | 14,675 | 294 | 3.20% |
| Alpha | Primary cycle | February 2021 to June 2021 | 150 | 1,717,350 | 11,449 | 39,263 | 262 | 2.29% |
| Delta | Primary cycle | July 2021 to September 2021 | 92 | 409,128 | 4,447 | 3,328 | 36 | 0.81% |
| Delta | Booster | October 2021 to December 2021 | 92 | 1,186,167 | 12,893 | 6,221 | 68 | 0.52% |
| Omicron BA.1/BA.2 | Booster | January 2022 to May 2022 | 150 | 11,542,295 | 76,949 | 29,540 | 197 | 0.26% |
| Omicron BA.4/BA.5 | Booster | June 2022 to present | 165 | 6,245,288 | 37,850 | 12,805 | 78 | 0.21% |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; VoC, variant of concern.
According to the official statistics of the Italian National Institute of Health (16), the number of estimated influenza-like illness cases was 21.3 million between the seasons 2013/14 and 2016/17, causing 68,068 attributable deaths, and thus yielding a crude death rate of 0.32% (Influenza virus vaccine coverage was between 50–65% during the same period) (16). Thus, the death rate of COVID-19 was approximately 11-fold higher than that of common flu at the beginning of the pandemic, but has now instead become nearly 36% lower after widespread COVID-19 vaccination and surge of Omicron sublineages BA.4/5 (Figure 1).
Discussion
There is now consolidated perception that after we have holistically learnt to live with common flu, it is very likely that we will now need to finally learn to live with SARS-CoV-2 as well, since this new coronavirus is on its way to become an(other) endemic human pathogen (19). Nonetheless, underrating the potentially detrimental clinical consequences of SARS-CoV-2 infections, or reclassifying COVID-19 “just like the flu” (13), may turn out to be paramount (even irreversible) mistakes, provided that reliable epidemiological data will be brought in support of this “attenuation and mitigation” thesis.
The evidence emerged from the analysis of Italian data about the progressive surge of SARS-CoV-2 VoCs combined with the progression of the nationwide COVID-19 vaccination campaign clearly attests that the lethality of COVID-19 had inexorably declined over time in the country (Figure 1), becoming over 17-fold lower in November 2022 than at the beginning of the national outbreak, nearly three years before (i.e., 0.21% vs. 3.53%). Notably, we have decided to arbitrarily limit our analysis to a single country (i.e., Italy), since the nationwide COVID-19 vaccination campaigns and the emergence and spread of new SARS-CoV-2 VoCs are both dramatically heterogeneous all around the world, thus precluding the possibility to pool data and obtain reliable information on these two different aspects on a global scale.
Looking at the temporal trend reported in Figure 1, it becomes evident that the initiation of the primary COVID-19 nationwide vaccination campaign in December 2020 in Italy has determined the sharpest decrease of the fatality rate, with continuation of such favorable trend when SARS-CoV-2 Alpha and Delta VoCs replaced the ancestral strain derived from the prototype virus firstly identified in Wuhan and bearing the lethal D614G polymorphism. At that point in time, the mortality rate had already declined to 0.81%. The effectiveness of the COVID-19 vaccine boosters administration has then maintained this favorable trend, being associated with a further reduction of the death rate by 1.36 folds (i.e., from 0.81% to 0.52%). Yet, such variation is not comparable to the magnitude of clinical benefits probably generated by the primary COVID-19 vaccination, which contributed to lower the mortality rate by over 4 folds between December 2020 and September 2021 (i.e., from 3.53% to 0.81%). This is in keeping with recent evidence showing that booster doses of COVID-19 vaccines may be effective to reduce the likelihood of developing acute SARS-CoV-2 infection by boosting humoral immunity, whilst both immunological memory (20) and cellular immunity (21,22) developed after primary vaccination remain almost unvaried (23), thus preserving part of their original potential to produce a marked anti-viral response that will ultimately prevent an unfavorable disease progression, including death (24).
Interestingly, the subsequent appearance and spread of the Omicron sublineages has then been associated with further reduction of the fatality rate, by exactly 2-fold during Omicron BA.1/2 preponderance (i.e., 0.26% versus 0.52% during the former Delta VoC wave) and by further 1.5-fold during the period of Omicron BA.4/5 surge (0.21% versus 0.26% during the former Omicron BA.1/2 wave), respectively. This epidemiological data are in keeping with the many biological studies published so far, reporting that the new Omicron sublineages are characterized by lower lethal potential on their human host, prevalently infecting the cells of the upper respiratory tract but exhibiting lower capacity to infect alveolar and inflammatory cells (25-28). Overall, our epidemiological results are also aligned to those earlier published by Lauring et al. in the US (29), who demonstrated that the clinical severity of COVID-19 has progressively declined over time across the country due to the combined effect of COVID-19 vaccination and virus attenuation.
Confronting the data garnered from our analysis with the recent death rate of common flu in that same country (i.e., 0.32%), it is then evident that SARS-CoV-2 was over tenfold more lethal than the Influenza virus at the beginning of the pandemic, but COVID-19 has now paradoxically become 36% less fatal than common flu at the beginning of November 2022 (Figure 1). Importantly, several lines of evidence attest that the efficacy of COVID-19 vaccination against symptomatic and severe COVID-19 illness slowly but progressively wanes over time (30), while the potential risk that more virulent and aggressive SARS-CoV-2 variants will emerge in the future cannot be discounted (31). Moreover, even if the clinical consequences of SARS-CoV-2 reinfection are milder than those caused by a primary infection, they are not clinically negligible, being associated with twofold higher risk of hospitalization and death compared to patients without reinfection (32). Irrespective of the favorable trend exhibited by the COVID-19 death rate recorded during the past three years, we hence proffer that it may be too premature to declass this new pathology to an influenza-like disease.
Conclusions
Although our data seem to underline a currently reassuring scenario in terms of COVID-19 death rate, we reemphasize the importance of preventing further surge of aggressiveness (and potential lethality) of SARS-CoV-2, especially in the most vulnerable parts of the population, which especially include unvaccinated, fragile and/or immunocompromised patients.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-75/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-75/coif). GL serves as the Editor-in-Chief of Journal of Laboratory and Precision Medicine. The other author has no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020;91:157-60. [PubMed]
- Sampath S, Khedr A, Qamar S, et al. Pandemics Throughout the History. Cureus 2021;13:e18136. [PubMed]
- Li Wan Po A. Omicron variant as nature's solution to the COVID-19 pandemic. J Clin Pharm Ther 2022;47:3-5. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Henry BM. COVID-19: unravelling the clinical progression of nature's virtually perfect biological weapon. Ann Transl Med 2020;8:693. [Crossref] [PubMed]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. Bethesda (MD): National Institutes of Health (US), 2021 Apr 21–2022 Sep 30.
- Mattiuzzi C, Henry BM, Lippi G. COVID-19 vaccination and SARS-CoV-2 Omicron (B.1.1.529) variant: a light at the end of the tunnel? Int J Infect Dis 2022;118:167-8. [Crossref] [PubMed]
- Barouch DH. Covid-19 Vaccines - Immunity, Variants, Boosters. N Engl J Med 2022;387:1011-20. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. Bilavent vaccines against COVID-19. Biochim Clin 2022; [Crossref]
- Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis 2022;22:1293-302. [Crossref] [PubMed]
- Lippi G, Henry BM. The landscape of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genomic mutations. J Lab Precis Med 2022;7:10. [Crossref]
- Mattiuzzi C, Henry BM, Lippi G. Regional Association between Mean Air Temperature and Case Numbers of Multiple SARS-CoV-2 Lineages throughout the Pandemic. Viruses 2022;14:1913. [Crossref] [PubMed]
- Fan Y, Li X, Zhang L, et al. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther 2022;7:141. [Crossref] [PubMed]
- Stokel-Walker C. How similar is covid-19 to the flu? BMJ 2022;379:o2625. [Crossref] [PubMed]
- World Health Organization. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/region/euro/country/it, last accessed November 11, 2022.
- Istituto Superiore di Sanità. COVID-19 integrated surveillance: key national data. Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data, last accessed November 11, 2022.
- Rosano A, Bella A, Gesualdo F, et al. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14-2016/17 seasons). Int J Infect Dis 2019;88:127-34. [Crossref] [PubMed]
- Stefanelli P, Trentini F, Petrone D, et al. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy, December 2021 to January 2022. Euro Surveill 2022; [Crossref] [PubMed]
- Italian Ministry of Health. COVID-19 Vaccines Report. Available online: https://www.governo.it/it/cscovid19/report-vaccini/, last accessed November 11, 2022.
- Lippi G, Mattiuzzi C, Henry BM. Updated picture of SARS-CoV-2 variants and mutations. Diagnosis (Berl) 2021;9:11-7. [Crossref] [PubMed]
- Kuse N, Zhang Y, Chikata T, et al. Long-term memory CD8(+) T cells specific for SARS-CoV-2 in individuals who received the BNT162b2 mRNA vaccine. Nat Commun 2022;13:5251. [Crossref] [PubMed]
- Takeuchi JS, Fukunaga A, Yamamoto S, et al. SARS-CoV-2 specific T cell and humoral immune responses upon vaccination with BNT162b2: a 9 months longitudinal study. Sci Rep 2022;12:15447. [Crossref] [PubMed]
- Oliver MA, Meredith RT, Smith BR, et al. Longitudinal T Cell Responses against Ancestral, Delta, and Omicron SARS-CoV-2 Variants Determined by Rapid Cytokine Release Assay in Whole Blood. Immunohorizons 2022;6:398-407. [Crossref] [PubMed]
- Maringer Y, Nelde A, Schroeder SM, et al. Durable spike-specific T-cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci Immunol 2022; Epub ahead of print. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C, Henry BM. Is cellular immunity the future key for deciphering and monitoring COVID-19 vaccines efficacy? J Lab Precis Med 2022;7:18. [Crossref]
- Hui KPY, Ng KC, Ho JCW, et al. Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract. EBioMedicine 2022;83:104232. [Crossref] [PubMed]
- Armando F, Beythien G, Kaiser FK, et al. SARS-CoV-2 Omicron variant causes mild pathology in the upper and lower respiratory tract of hamsters. Nat Commun 2022;13:3519. [Crossref] [PubMed]
- McMahan K, Giffin V, Tostanoski LH, et al. Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters. Med (N Y) 2022;3:262-268.e4. [Crossref] [PubMed]
- Granerud BK, Ueland T, Lind A, et al. Omicron Variant Generates a Higher and More Sustained Viral Load in Nasopharynx and Saliva Than the Delta Variant of SARS-CoV-2. Viruses 2022; [Crossref] [PubMed]
- Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022;376:e069761. [Crossref] [PubMed]
- Ferdinands JM, Rao S, Dixon BE, et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ 2022;379:e072141. [Crossref] [PubMed]
- Caputo E, Mandrich L. SARS-CoV-2: Searching for the Missing Variants. Viruses 2022;14:2364. [Crossref] [PubMed]
- Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med 2022;28:2398-405. [Crossref] [PubMed]
Cite this article as: Mattiuzzi C, Lippi G. Nationwide analysis of COVID-19 death rate throughout the pandemic in Italy. J Lab Precis Med 2023;8:3.


