Circulating forms of cardiac troponin: a review with implications for clinical practice
Introduction
Background
Cardiac troponin (cTn) measurement in blood is one of the cornerstones in diagnosing myocardial infarction (MI) (1). Due to the fact that cTnI and cTnT are solely expressed in cardiomyocytes and consist of a unique, cardiac-specific amino acid sequence, they both became the preferred biomarkers for detecting acute MI or other causes of myocardial injury (2). Currently, both cTnI and cTnT are considered equally good as the diagnostic performance of these biomarkers is comparable (3,4). Clinical laboratories usually implement one cTn immunoassay, which in practice depends on the vendor of a clinical chemistry analyzer used within the clinical laboratory (3).
The introduction of high-sensitivity (hs-)cTn immunoassays allowed a more accurate assessment of cTn concentrations in the lower analytical range (5). This led to a substantial rise in the number of patients diagnosed with non-ST-elevated MI (NSTEMI) and reduced the portion of patients classified as having unstable angina pectoris (6,7). However, the downside of these hs-cTn immunoassays is the frequent detection of elevated hs-cTnI and hs-cTnT concentrations in conditions other than acute MI (6,8,9). Additionally, hs-cTn elevations were more frequently observed in non-cardiac pathologies and even in presumed physiological conditions such as vigorous exercise (6,10). Though the hs-cTn immunoassay algorithms have excellent negative predictive values (NPVs) for acute MI rule-out (≥99%), their positive predictive values (PPVs) for acute MI rule-in remains suboptimal (75–80%) (11). In the United States, the PPV may even be significantly lower due to the lower prevalence of MI patients in chest pain cohorts (12). This limitation in clinical specificity complicates clinical decision making on a daily basis and new strategies to overcome this issue are highly warranted.
Previous research illustrated different circulating cTnI and cTnT forms in the blood circulation (13-18). Interestingly, some of these cTn forms in the blood follow a time-dependent pattern that seems to be based on symptom onset of acute chest pain. Further knowledge of circulating cTn forms, and acute cTn forms in particular, might therefore be of clinical relevance in the search of enhancing the clinical specificity of hs-cTn immunoassays (16,19-21).
Rationale and knowledge gap
Previous reviews from Mair et al. (21) and Hammarsten et al. (22) mainly described mechanisms of cTn release from injured myocardium, while a recent review from Katrukha et al. (23) described mechanisms of cTn release in MI, cleavage sites of cTn, and different forms of cTn in MI. Our review adds to this knowledge by also describing the various techniques currently available for analyzing cTn forms, and combining this with previous research on cTn forms in various populations [e.g., end-stage renal disease (ESRD) patients and after vigorous exercise] and the effect of the blood tube on the cTn forms. This might serve as a stepping stone towards new hs-cTn immunoassay generations more specific for acute MI.
Objective
The objective of this review is to describe the most commonly used analytical techniques for studying circulating cTn forms and provide an overview of research on the circulating cTn forms performed thus far.
Analytical techniques to study circulating forms of cTn
Several customized analytical methods were developed by different research groups to study circulating cTn forms in blood: (I) gel filtration chromatography (GFC); (II) immunoprecipitation followed by Western blotting (WB); and (III) custom-made immunoassays against specific cTn forms (13,16,17,24-27). Since GFC and WB provide complementary information on the cTn composition, this is explained in further detail in section “GFC versus WB”.
GFC
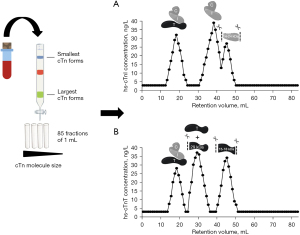
GFC is a technique used to separate cTn based on size exclusion and without losing their native three-dimensional molecular structure (Figure 1) (13,16,17,24-26,28). In short, blood is loaded on a GFC column that is equilibrated with a physiological running buffer and enriched with albumin to mimic the protein-like environment from blood plasma or serum. After loading, larger cTn forms cannot enter the porous GFC beads and will elute first, while smaller cTn forms will be slowed down and elute last. Subsequently, GFC elution profiles are established by collecting fractions and assessing the cTnI and/or cTnT concentrations in each fraction. To validate for run-to-run variation so called internal serum or plasma standards have been identified that are present in typical blood samples and they should elute at certain expected elution volumes (within <2 mL). Albumin, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and/or myoglobin are examples of such internal standards. Also, calibration and validation of the GFC set-up is performed using dextran blue (void volume), globular calibration proteins (optional) and purified cTn standards (available either in complex or free). Importantly, elution of cTn proteins clearly deviates from typical globular proteins (14). To our experience, the detection limit for cTnI is approximately 20 ng/L (Abbott Diagnostics, Alinity), while for cTnT it is feasible to characterize circulating forms in blood with a minimal cTnT concentration of approximately >60 ng/L.
Immunoprecipitation and WB
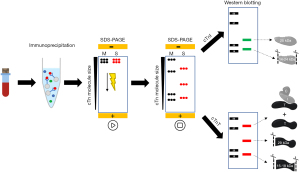
Immunoprecipitation is a technique to purify and concentrate low amounts of cTn protein from blood which is subsequently characterized for its molecular weight by WB analysis. Streptavidin-coated magnetic beads coupled to a specific biotinylated antibody against cTnI or cTnT are used because of the strong streptavidin-biotin affinity, leading to yields of >80%. Depending on the epitope of the chosen antibody, particular cTn forms will bind to the antibody on the beads, after which the remaining supernatant will be discarded and the cTn protein will be eluted from the beads. Next, gel electrophoresis will be used to separate the cTn forms from each other based on their molecular weight. Sodium dodecyl sulfate (SDS) is added to the eluted sample and a negative charge is applied to a polyacrylamide gel, which will separate the cTn forms present in the sample. Next, the proteins are transferred to a membrane and WB is used to specifically detect cTn proteins. For instance, first a primary antibody is used to detect the cTn protein, while the secondary antibody is added to visualize the protein by emitting a fluorescent signal (Figure 2). Many attempts were performed to lower the detection limit of this set-up as much as possible, for instance by increasing sample volumes and incubation times, though the detection limit for WB is still relatively high: for cTnI this has not been described, for cTnT the detection limit for WB is in our experience approximately 300 ng/L, as verified in Mingels et al. (16).
GFC versus WB
With regards to the cTn composition, GFC is an essential technique to study cTn complexes (cTn T-I-C and cTn I-C) as these cannot be studied by WB due to the addition of SDS which disintegrates the different cTn complexes into the different cTn subunits. In contrast, the GFC technique is not sensitive enough to separate the intact cTnI and cTnT subunits from (some of) its fragments, e.g., intact cTnT and its 29 kDa fragment elute approximately at the same retention volume. The WB technique is able to differentiate the different intact cTn from its fragments as it separates protein (fragments) based on their molecular weight. Hence, GFC and WB are considered as complementary techniques to study the cTn composition.
Immunoassays against specific cTn forms
Immunoassays are based on the very unique antibody—antigen recognition, similar to a key and lock principle. Clinical cTn immunoassays often contain two to four monoclonal antibodies, based on a combination of both detection and capture antibodies, that are targeted against specific regions of the cTn protein (epitopes). The detection antibody is labeled with a signaling tag and the amount of signal is equal to the cTn concentration in a blood sample (29). For cTnI and cTnT several antibodies are (commercially) available that are directed against specific regions in the cTn protein.
Two research groups used these different antibodies to design various immunoassays against various cTn forms (26,27). Vylegzhanina et al. designed an immunoassay to detect cTnI in complex with TnC, and in complex with both TnC and cTnT. Their second immunoassay detected free cTnT and cTnT in complex with TnC and cTnI, while the third immunoassay only targeted cTnI when in complex with TnC, but not in complex with both TnC and cTnT. They also experimented with “mixed sandwich assays” in which the detection and capture antibodies targeted different components of the cTn T-I-C complex, which allowed them to develop immunoassays against the various cTn complexes (26). Damen et al. also developed three assays to determine the circulating cTn forms in NSTEMI patients. One assay detected total cTnI (both complexed and non-complexed forms), the second assay targeted complexed cTnI (full-size, partially truncated, and low molecular weight cTn T-I-C complex and cTn I-C complex), while the third assay was designed to only detect large-size cTn T-I-C complexes (full-size and partially truncated cTn T-I-C complex) (27).
Circulating cTn forms
The ternary cTn T-I-C complex has a crucial role in the contractile apparatus of cardiomyocytes, when thick (myosin) and thin (actin) filaments slide along each other upon muscle contraction and relaxation. cTn is anchored on top of tropomyosin and consists of three subunits; cTnI (inhibitory function to myosin-actin binding site blockage), cTnT (connection of the cTn complex to tropomyosin), and the non-cardiospecific subunit troponin C (TnC; calcium binding) (25,26). After calcium enters the cell and binds to TnC, a conformational change happens where cTnI loses its inhibitory function and muscle contraction is initiated. After myocardial injury, the cardiomyocytes will release cTn into the blood circulation in a variety of different cTn forms.
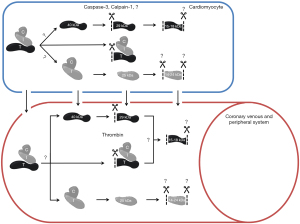
Studies investigating circulating cTn forms have shown that both cTnI and cTnT are susceptible to degradation by intra- and extracellular proteases. This results in the presence of various forms of cTn within the circulation after myocardial injury (Figure 3) (14,30-33).
Circulating cTnI forms in MI
Katrukha et al. used several monoclonal cTnI antibodies for WB to investigate which forms of cTnI are present in serum samples of 30 acute MI patients (34). cTnI was mainly found to be present in a binary complex, most probably with TnC, while only a small portion of free cTnI was observed. To study the cTnI composition, Wu et al. included several commercial and pre-commercial cTnI immunoassays. They concluded that mainly the cTn I-C complex and ternary cTn T-I-C complex in serum of three acute MI patients were detected (25). More recently, both Giuliani et al. and Damen et al. investigated the forms of cTnI present in serum of acute MI patients by the development of three sandwich immunoassays (27,35). Giuliani et al. observed that the cTn I-C complex was predominantly present, while only small amounts of free cTnI, cTn I-T complex, and cTn T-I-C complex were detected (35). Damen et al. observed that the concentration of large-size cTn T-I-C complexes and cTn I-C complex was higher in the coronary veins compared to the peripheral circulation (27). When comparing early- and late-symptomatic MI patients, they did not find a significant difference for the percentage of complex cTnI (relative to total cTnI concentration), while a significant difference was found when looking at the proportion of large-size cTn T-I-C complex. Based on all these findings, circulating cTnI in serum of acute MI patients seems mainly present in the blood circulation in the cTn I-C complex and to a lesser extent also in cTn T-I-C complexes.
Despite this, it is generally accepted that cTnI is highly susceptible to degradation. An early study performed by Morjana in 1998 observed that serum cTnI in acute MI patients is present as two major fragments with apparent molecular masses of 14 and 18 kDa using SDS-PAGE and WB (36). A study performed in 2006 by Madsen et al. revealed the presence of up to 7 cTnI fragments in serum samples of patients with ST-elevation myocardial infarction (STEMI) ranging from 12 to 20 kDa (intact cTnI was considered 24 kDa) (37). Besides, they defined for the first time the initial time course of cTnI degradation in those patients. As early as 90 minutes after onset of symptoms, intact cTnI and a single degradation product of 20 kDa was detectable, with further degradation to multiple fragments after 165 minutes (37). A more recent study of Katrukha et al. revealed the presence of 11 cTnI fragments in serum of STEMI patient ranging from 14 to 28 kDa (intact cTnI was considered 29 kDa), while no time-dependent cTnI degradation was observed. They indeed stated that their results on time-dependent cTnI degradation contradicted the study of Madsen et al. (18,37). They attributed this contradiction to the sensitivity of the detection system, as the cTnI concentration after 90 minutes was thought to be too low to detect cTnI fragments. A later study by Madsen et al. also showed no time-dependent cTnI degradation (38). A study by Vylegzhanina et al. showed that cTn T-I-C complex might degrade into low molecular weight cTn T-I-C complex and IC complex over time. It might therefore be of interest to compare the relative amount of cTn T-I-C complex in acute MI compared to other (non-)cardiac pathofysiologies (26). Furthermore, a study by Zahran et al. did suggest that the degree of proteolytic cTnI digestion increased with the severity of the myocardial injury, however, whether this finding can result in a specific tool for acute myocardial injury remains to be seen (39).
The exact borders of the observed cTnI fragments have not been fully characterized, but the most stable part of cTnI is approximately demarcated by amino acid 34–126 (18). To our knowledge, no studies have investigated the cTnI composition in other pathologies yet. It may be interesting to investigate this in order to assess the differentiating ability of the cTnI composition for diagnosing MI. But the question remains whether this is useful as cTnI does not seem to undergo time-dependent degradation.
Circulating cTnT forms in MI
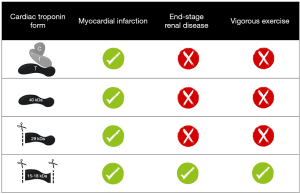
Research has demonstrated that cTnT is present in the circulation after acute MI as part of the ternary cTn T-I-C complex, as free subunit and smaller fragments (25,26). Regarding cTnT, fragments of 29 and 15–18 kDa have been characterized in the blood circulation (16). The 29 kDa fragment is formed by cleavage at the N-terminal end, while 15–18 kDa cTnT fragments are formed by additional truncation of the C-terminal end (Figure 4) (40). In contrast to cTnI, the circulating cTnT composition in patients with acute MI appeared to be time-dependent, with a higher percentage of larger cTnT forms (cTn T-I-C and as free subunit) being actively released at early presentation as compared to predominantly cTnT fragments in late presenters (13,14). This finding might suggest that the size of the cTnT form might reflect the state of the injured myocardium and serve as marker of the age of the infarction. This could potentially aid in the selection of patients with uncertainty regarding symptom onset and whom could still benefit from an early invasive strategy. Currently, all cTnT forms are detected by the fifth generation hs-cTnT immunoassay (Roche Diagnostics) since it targets two epitopes located in the stable middle region of the cTnT protein (Figure 4) (5).
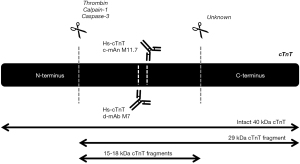
Circulating cTnT forms in other conditions
It is common knowledge that hs-cTnT concentrations are often elevated in other non-MI populations, mostly patients at cardiac risk, for example ESRD patients, but elevated concentrations are also measured in marathon runners after vigorous exercise (1,16,17,41). When circulating cTnT forms were characterized in serum of ESRD patients (n=16), both pre- and post-hemodialysis using GFC and WB analysis, it was revealed that at baseline and after 2 months follow-up only 15–18 kDa cTnT fragments were observed (16). Likewise, in serum of marathon runners GFC analysis also revealed only 15–18 kDa cTnT fragments of which the N-terminal and C-terminal ends of the protein were cleaved off (17). The circulating cTnT form in marathon runners postrace seems identical to the form seen in ESRD patients, as far as this could be determined with WB (17). Several mechanisms have been proposed for cTnT elevations after vigorous exercise, i.e., physiologic remodeling of the myocardium, increased cardiac workload, or “bleb/vesicle” formation caused by transient ischemia (41,42). However, more definitive insight in the mechanism(s) for cTn release and degradation in both vigorous exercise and ESRD is required before attempting to explain why both vigorous exercise and ESRD appear to have identical cTnT forms. Though, the cTn kinetics in ESRD and vigorous exercise is completely different, with chronic cTnT elevations in ESRD and a rapid increase and decrease in vigorous exercise (1,41). The cTnT composition in other cardiovascular diseases associated with hs-cTnT elevations, including atrial fibrillation, heart failure, and cardiomyopathies amongst others, still remains to be investigated, and similarly holds true for more systemic diseases like sepsis and pre-eclampsia. These findings suggest that circulating cTnT forms in acute MI differ from other (patho)physiologies associated with hs-cTnT elevations (Figure 5) (13,15-17).
Hence, it has been suggested that the diagnostic specificity of the hs-cTnT immunoassay for acute MI might be enhanced (i.e., increasing the PPV while maintaining a high NPV) by exclusively targeting cTnT forms of ≥29 kDa (19,21). This more specific hs-cTnT immunoassay could act as an additional simultaneously performed test to further specify the cause of the cTnT elevations, without missing late or delayed acute MI. Especially in patients with an atypical or dubious MI diagnosis, it ideally might be that such information could prevent unnecessary coronary angiographies.
Influence of proteases on circulating cTnT forms
Both intracellular and extracellular proteases were suggested to cause cTnT degradation (Figure 3) (31,32,43-45). The most prominent suggested intracellular cTnT proteases are calpain-1 and caspase-3. Physiologically, calpain-1 is involved in the cardiomyocyte protease system essential for protein quality control to maintain cardiac function, while caspase-3 functions as a mediator in cardiomyocyte apoptosis (44,45). Both calpain-1 and caspase-3 can cleave cTnT at the N-terminal region, Arg68-Ser69 and between Asp88-Asp89 respectively (30,45,46). In addition, also extracellular thrombin has been identified to cleave the N-terminal region of cTnT, which coincidentally happens at the same side as cleavage of calpain-1 (31,32). Moreover, truncation at the C-terminus is required for the formation of the smallest 15–18 kDa cTnT fragments. Thus far, no protease capable of C-terminal cTnT truncation has been identified. Nonetheless, if there are differences in the mechanisms underlying cTnT release between different etiologies, it is to be expected that different proteases are upregulated within the cardiomyocytes. Hypothetically, this could result in disease-specific circulating cTnT compositions. In turn, further research towards cTnT composition in situations in which cardiomyocyte necrosis is highly unlikely, such as vigorous exercise (41), could maybe elucidate new (patho)physiological mechanisms underlying cTn release.
Influence of blood tubes on circulating cTnT forms
Several blood tubes are allowed by the manufacturer for hs-cTnT concentration assessment, namely lithium-heparin (LH) and EDTA plasma and serum, as is described in package inserts and as is validated in research studies with perfect correlations (r>0.9) (5). However, it is important to realize that the concentration and activity of thrombin is significantly different between these blood tubes (31,32). Indeed, in simultaneously collected LH-plasma and serum samples (5), it became evident that predominantly intact cTnT was observed in LH plasma, compared to abundant 29 and 15–18 kDa cTnT fragments in serum (32). This effect was attributed to thrombin, which is abundantly generated during serum production, causing pre-analytical cTnT degradation (31,32). Hence, LH plasma was suggested to better represent the in vivo situation and therefore seems the preferred blood matrix when characterizing circulating cTn forms.
Strengths and limitations
This review provides an extensive and focused overview on circulating cTn forms for both cTnI and cTnT. This review not only described cTn forms in MI, but also included other cardiac pathofysiologies, and non-cardiac conditions and the potential clinical relevance of these cTn forms. Worldwide, this research topic is only studied by a few research groups limiting the availability of studies needed for a systematic review.
Conclusions
In acute MI patients, cTnI appears to be mostly present as part of the cTn I-C. Furthermore, a variety of different cTnI fragments have been identified, but its degradation does not seem to be time-dependent. More research into the cTnI composition in other conditions associated with hs-cTnI elevations is warranted.
With respect to cTnT, its composition in acute MI is different from the cTnT composition as observed in several other conditions associated with hs-cTnT elevations. Based on this preliminary data it was suggested that developing a novel hs-cTnT immunoassay which solely targets specific cTnT forms only observed in acute MI could help to further increase the specificity of hs-cTnT immunoassay for acute MI.
Hence, studying circulating forms of cTn with techniques such as GFC and immunoprecipitation followed by WB in multiple conditions associated with hs-cTn elevations might serve useful in search of enhancing the clinical specificity of hs-cTn immunoassays for acute MI (12-15).
Acknowledgments
Funding: This work was supported by Veni grant (No. 09150161810155).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Xander van Wijk, Amy Saenger, Steven Meex, and Allan Jaffe) for the series “Cardiac Troponin” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-66/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-66/coif). The series “Cardiac Troponin” was commissioned by the editorial office without any funding or sponsorship. AM receives support from Dutch government, Nederlandse organisatie voor gezondheidsonderzoek en zorginnovatie/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Case 09150161810155) and a grant from Academic Alliance Fund Maastricht UMC-Radboudmc, The Netherlands (Case SSC 154.2021). AM receives support from Roche Diagnostics for travelling and has no financial support from Roche Diagnostics and Abbott Diagnostics about the receipt of equipment. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018;138:e618-51. [Crossref] [PubMed]
- Burlina A, Zaninotto M, Secchiero S, et al. Troponin T as a marker of ischemic myocardial injury. Clin Biochem 1994;27:113-21. [Crossref] [PubMed]
- van Doorn WPTM, Vroemen WHM, de Boer D, et al. Clinical laboratory practice recommendations for high-sensitivity cardiac troponin testing. J Lab Precis Med 2018;3:30. [Crossref]
- Rubini Gimenez M, Twerenbold R, Reichlin T, et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J 2014;35:2303-11. [Crossref] [PubMed]
- Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254-61. [Crossref] [PubMed]
- Giannitsis E, Katus HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol 2013;10:623-34. [Crossref] [PubMed]
- Reichlin T, Twerenbold R, Reiter M, et al. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med 2012;125:1205-1213.e1. [Crossref] [PubMed]
- Westermann D, Neumann JT, Sörensen NA, et al. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol 2017;14:472-83. [Crossref] [PubMed]
- Smulders MW, Kietselaer BL, Schalla S, et al. Acute chest pain in the high-sensitivity cardiac troponin era: A changing role for noninvasive imaging? Am Heart J 2016;177:102-11. [Crossref] [PubMed]
- Aakre KM, Omland T. Physical activity, exercise and cardiac troponins: Clinical implications. Prog Cardiovasc Dis 2019;62:108-15. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- McCord J, Hana A, Cook B, et al. The role of cardiac testing with the 0/1-hour high-sensitivity cardiac troponin algorithm evaluating for acute myocardial infarction. Am Heart J 2021;233:68-77. [Crossref] [PubMed]
- Damen SAJ, Vroemen WHM, Brouwer MA, et al. Multi-Site Coronary Vein Sampling Study on Cardiac Troponin T Degradation in Non-ST-Segment-Elevation Myocardial Infarction: Toward a More Specific Cardiac Troponin T Assay. J Am Heart Assoc 2019;8:e012602. [Crossref] [PubMed]
- Cardinaels EP, Mingels AM, van Rooij T, et al. Time-dependent degradation pattern of cardiac troponin T following myocardial infarction. Clin Chem 2013;59:1083-90. [Crossref] [PubMed]
- Eijsvogels TM, Shave R, van Dijk A, et al. Exercise-induced cardiac troponin release: real-life clinical confusion. Curr Med Chem 2011;18:3457-61. [Crossref] [PubMed]
- Mingels AM, Cardinaels EP, Broers NJ, et al. Cardiac Troponin T: Smaller Molecules in Patients with End-Stage Renal Disease than after Onset of Acute Myocardial Infarction. Clin Chem 2017;63:683-90. [Crossref] [PubMed]
- Vroemen WHM, Mezger STP, Masotti S, et al. Cardiac Troponin T: Only Small Molecules in Recreational Runners After Marathon Completion. J Appl Lab Med 2019;3:909-11. [Crossref] [PubMed]
- Katrukha IA, Kogan AE, Vylegzhanina AV, et al. Full-Size Cardiac Troponin I and Its Proteolytic Fragments in Blood of Patients with Acute Myocardial Infarction: Antibody Selection for Assay Development. Clin Chem 2018;64:1104-12. [Crossref] [PubMed]
- deFilippi C, Seliger S. The Cardiac Troponin Renal Disease Diagnostic Conundrum: Past, Present, and Future. Circulation 2018;137:452-4. [Crossref] [PubMed]
- Mair J, Lindahl B, Müller C, et al. What to do when you question cardiac troponin values. Eur Heart J Acute Cardiovasc Care 2018;7:577-86. [Crossref] [PubMed]
- Mair J, Lindahl B, Hammarsten O, et al. How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care 2018;7:553-60. [Crossref] [PubMed]
- Hammarsten O, Mair J, Möckel M, et al. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018;23:725-34. [Crossref] [PubMed]
- Katrukha IA, Katrukha AG. Myocardial Injury and the Release of Troponins I and T in the Blood of Patients. Clin Chem 2021;67:124-30. [Crossref] [PubMed]
- Bates KJ, Hall EM, Fahie-Wilson MN, et al. Circulating immunoreactive cardiac troponin forms determined by gel filtration chromatography after acute myocardial infarction. Clin Chem 2010;56:952-8. [Crossref] [PubMed]
- Wu AH, Feng YJ, Moore R, et al. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. American Association for Clinical Chemistry Subcommittee on cTnI Standardization. Clin Chem 1998;44:1198-208. [Crossref] [PubMed]
- Vylegzhanina AV, Kogan AE, Katrukha IA, et al. Full-Size and Partially Truncated Cardiac Troponin Complexes in the Blood of Patients with Acute Myocardial Infarction. Clin Chem 2019;65:882-92. [Crossref] [PubMed]
- Damen SAJ, Cramer GE, Dieker HJ, et al. Cardiac Troponin Composition Characterization after Non ST-Elevation Myocardial Infarction: Relation with Culprit Artery, Ischemic Time Window, and Severity of Injury. Clin Chem 2021;67:227-36. [Crossref] [PubMed]
- Duong-Ly KC, Gabelli SB. Gel filtration chromatography (size exclusion chromatography) of proteins. Methods Enzymol 2014;541:105-14. [Crossref] [PubMed]
- Radha R, Shahzadi SK, Al-Sayah MH. Fluorescent Immunoassays for Detection and Quantification of Cardiac Troponin I: A Short Review. Molecules 2021;26:4812. [Crossref] [PubMed]
- Di Lisa F, De Tullio R, Salamino F, et al. Specific degradation of troponin T and I by mu-calpain and its modulation by substrate phosphorylation. Biochem J 1995;308:57-61. [Crossref] [PubMed]
- Streng AS, de Boer D, van Doorn WP, et al. Cardiac troponin T degradation in serum is catalysed by human thrombin. Biochem Biophys Res Commun 2016;481:165-8. [Crossref] [PubMed]
- Katrukha IA, Kogan AE, Vylegzhanina AV, et al. Thrombin-Mediated Degradation of Human Cardiac Troponin T. Clin Chem 2017;63:1094-100. [Crossref] [PubMed]
- Mahmud Z, Zahran S, Liu PB, et al. Structure and proteolytic susceptibility of the inhibitory C-terminal tail of cardiac troponin I. Biochim Biophys Acta Gen Subj 2019;1863:661-71. [Crossref] [PubMed]
- Katrukha AG, Bereznikova AV, Esakova TV, et al. Troponin I is released in bloodstream of patients with acute myocardial infarction not in free form but as complex. Clin Chem 1997;43:1379-85. [Crossref] [PubMed]
- Giuliani I, Bertinchant JP, Granier C, et al. Determination of cardiac troponin I forms in the blood of patients with acute myocardial infarction and patients receiving crystalloid or cold blood cardioplegia. Clin Chem 1999;45:213-22. [Crossref] [PubMed]
- Morjana NA. Degradation of human cardiac troponin I after myocardial infarction. Biotechnol Appl Biochem 1998;28:105-11. [PubMed]
- Madsen LH, Christensen G, Lund T, et al. Time course of degradation of cardiac troponin I in patients with acute ST-elevation myocardial infarction: the ASSENT-2 troponin substudy. Circ Res 2006;99:1141-7. [Crossref] [PubMed]
- Madsen LH, Lund T, Grieg Z, et al. Cardiac troponin I degradation in serum of patients with hypertrophic obstructive cardiomyopathy undergoing percutaneous septal ablation. Cardiology 2009;114:167-73. [Crossref] [PubMed]
- Zahran S, Figueiredo VP, Graham MM, et al. Proteolytic Digestion of Serum Cardiac Troponin I as Marker of Ischemic Severity. J Appl Lab Med 2018;3:450-5. [Crossref] [PubMed]
- Streng AS, de Boer D, van Doorn WP, et al. Identification and Characterization of Cardiac Troponin T Fragments in Serum of Patients Suffering from Acute Myocardial Infarction. Clin Chem 2017;63:563-72. [Crossref] [PubMed]
- Aengevaeren VL, Baggish AL, Chung EH, et al. Exercise-Induced Cardiac Troponin Elevations: From Underlying Mechanisms to Clinical Relevance. Circulation 2021;144:1955-72. [Crossref] [PubMed]
- Gresslien T, Agewall S. Troponin and exercise. Int J Cardiol 2016;221:609-21. [Crossref] [PubMed]
- Katrukha AG, Bereznikova AV, Filatov VL, et al. Degradation of cardiac troponin I: implication for reliable immunodetection. Clin Chem 1998;44:2433-40. [Crossref] [PubMed]
- Ke L, Qi XY, Dijkhuis AJ, et al. Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation. J Mol Cell Cardiol 2008;45:685-93. [Crossref] [PubMed]
- Communal C, Sumandea M, de Tombe P, et al. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A 2002;99:6252-6. [Crossref] [PubMed]
- Streng AS, de Boer D, van der Velden J, et al. Posttranslational modifications of cardiac troponin T: an overview. J Mol Cell Cardiol 2013;63:47-56. [Crossref] [PubMed]
Cite this article as: Denessen EJS, Nass SIJ, Bekers O, Vroemen WHM, Mingels AMA. Circulating forms of cardiac troponin: a review with implications for clinical practice. J Lab Precis Med 2023;8:13.