
Circulating levels of lipoprotein lipase and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1: new markers for cardiovascular diseases among noncommunicable diseases: a brief narrative review
Introduction
Noncommunicable diseases (NCDs) account for roughly three-quarters (74%) of all deaths worldwide. The most common NCDs are cancers, diabetes, chronic lung disease, and heart disease. Metabolic risk factors, such as overweight/obesity, high blood pressure, hyperglycemia, and hyperlipidemia increase the risk of NCDs (1). An increased risk of atherosclerotic cardiovascular events is associated with high levels of triglyceride-rich lipoprotein (TRL) remnants derived from hepatic and intestinal sources (2). Increased levels of circulating TRLs, such as chylomicrons (CMs) and very low-density lipoproteins (VLDLs), exacerbate cardiovascular disease by promoting atherosclerosis (3). Most conventional triglyceride-lowering therapies do not reduce the risk of cardiovascular events in statin-treated patients; however, in patients with varying triglyceride levels and experimental models, new treatment modalities that target catalytic pathways in TRL metabolism decrease TRL concentrations and atherosclerosis (2-4). These studies may lead to the development of new therapies that reduce TRL levels and cardiovascular risk (2). The majority of new therapeutic targets regulate lipoprotein lipase (LPL) activity (2,5,6). LPL is an important player in TRL metabolism (7); however, the actual clinical significance of pre-heparin LPL mass and the relationship between circulating LPL levels and NCDs remains unknown. In this review, we updated the clinical significance of determining LPL concentration in pre-heparin serum. We present this article in accordance with the Narrative Review reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-12/rc).
Methods
We used the following search terms to find articles published in English in the Cochrane Central, EMBASE, MEDLINE, PubMed, and Web of Science databases: triglyceride; lipoprotein lipase (LPL); glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1); chylomicron (CM); very low-density lipoprotein (VLDL); heparin; noncommunicable disease; insulin resistance; diabetes mellitus; pre-diabetes; cardiovascular disease; diagnosis; and prognosis. All authors compiled the final reference list after independently selecting articles and evaluating data quality, presentation, and interpretation in light of the study’s central idea (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | December 1 to 31, 2022 |
| Databases and other sources searched | PubMed |
| Search items used | “Triglyceride”; “LPL”; “GPIHBP1”; “CM”; “VLDL”; “LDL”; “Heparin”; “Noncommunicable Disease”; “Insulin Resistance”; “Diabetes Mellitus”; “Pre-Diabetes”; “Cardiovascular Disease”; “Diagnosis”; “Prognosis” |
| Timeframe | January 1943 to December 2022 |
| Inclusion criteria | English text; human and animal investigation |
| Selection process | All authors selected and had consensus |
LPL, lipoprotein lipase; GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1; CM, chylomicron; VLDL, very low-density lipoprotein; LDL, low-density lipoprotein.
LPL
LPL hydrolyzes triglyceride and acts as a ligand
LPL was discovered in 1943 as a heparin-activated clearing factor (8) and renamed LPL in 1955 (9,10). LPL is a 50 kDa protein that hydrolyzes triglycerides in circulating CMs and VLDL on vascular endothelial cell surfaces (11). LPL-catalyzed lipolysis of TRLs by LPL is the rate-limiting step in triglyceride clearance from the blood, making it an important process in lipid metabolism. Natural lipolysis by LPL results in the release of fatty acids for tissue uptake, the production of low-density lipoprotein (LDL), and the elevation of high-density lipoprotein (HDL) (12). LPL is transported to the surfaces of vascular endothelial cell surfaces from its primary sites of production in the heart, adipose tissues, and skeletal muscle (13-15). LPL mass detaches from the vascular endothelial surface and is carried to the liver for elimination as it degrades (16,17). Although LPL mass exists in pre-heparin serum, LPL activity is rare (in the absence of intravascular heparin injection) (16,17). LPL, which is catalytically inactive, mediates lipoprotein metabolism in the liver for lipoprotein receptors and glucosaminoglycans via its ligand function rather than its lipolytic function (18-22). Inactive LPL promotes the uptake of cholesteryl ester and VLDL into cells and organs. This results in decreased VLDL triglycerides (22). However, because serum pre-heparin LPL is catalytically inactive, measuring pre-heparin LPL concentration has not been widely studied as a diagnostic marker (23).
Regulatory mechanism of triglyceride lipolysis by LPL
Recent reviews summarized the regulatory mechanisms of intravascular lipolytic processing of TRLs by LPL along the luminal surface of capillaries (11,24). LPL activity is tightly regulated at the transcriptional, post-transcriptional, translational, and post-translational levels because of its critical role in lipid homeostasis (25,26). Several proteins, including apolipoprotein (apo)C1 (27,28), apoC2 (29), apoC3 (28,30), apoA5 (31), angiopoietin-like protein 3 (ANGPTL3) (32), ANGPTL4 (33,34), and ANGPTL8, regulate LPL (35). LPL is synthesized and secreted as a monomer rather than a homodimer from head-to-tail (36,37). To preserve its native fold, LPL must be chaperoned in all compartments because it is inherently unstable (38). LPL is chaperoned in the endoplasmic reticulum by lipase maturation factor 1 (LMF1) and Sel-1 suppressor of Lin-12-like 1 (Sel1L) during parenchymal cell biosynthesis. LPL is chaperoned by heparan sulfate-modified syndecan-1 (SDC1) as it moves from the trans-Golgi network into the secretory pathway (39). Heparan sulfate proteoglycans (HSPGs) in the extracellular matrix and the glycocalyx of parenchymal cells regulate LPL in the subendothelial space. GPIHBP1 transports LPL from the abluminal endothelial surface to its site of action in the capillary lumen (11,40-42). GPIHBP1, an effective chaperone for LPL, maintains its native and active states (43). The acidic domain increases the rate of GPIHBP1 and LPL association by 2,500-fold, allowing LPL to transition from an HSPG-bound to a GPIHBP1-bound state and then enter the capillary lumen via transcytosis (44). LPL is stabilized by binding to TRLs; however, apoC1 and apoC3 displace LPL from lipid droplets (LDs) (26). Angiopoietin-like protein (ANGPTL)-3, -4, and -8 inhibits LPL activity by converting stable LPL dimers to unstable monomers. By binding directly to LPL monomers, ANGPTL4 catalyzes the irreversible unfolding of LPL’s α/β-hydrolase domain (37,38,44,45). GPIHBP1 binding to LPL prevents this inhibition. An ANGPTL3/ANGPTL8 oligomeric complex regulates LPL activity in oxidative tissues (46-51). Therapeutic strategies that improve LPL function, decrease apoC3 and ANGPTL4 function, or increase apoA5 function are expected to have cardioprotective effects (26). Genetic alterations affecting LPL activity are summarized by Shaik et al. LPL activity is elevated by loss of function of apoC3, ANGPTL3 and ANGPTL4 and decreased by loss of function of apoA5 (2,3) (Figure 1).
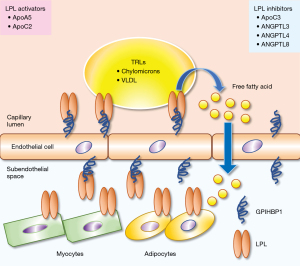
LPL activity in the fasted and fed state
Kristensen et al. indicated fasting- and fed-state LPL activity (24). During fasting or exercise, TRLs must be directed away from storage in white adipose tissue (WAT) and toward oxidative tissues such as the heart and skeletal muscles. This is accomplished by (I) increasing the expression of ANGPTL4 in WAT, which inhibits LPL secretion and inactivates LPL in the subendothelial space, and (II) downregulation of hepatic ANGPTL8 expression, which significantly reduces the effectiveness of ANGPTL3-mediated LPL inhibition (46,52,53). The TRL flux must quickly switch from oxidative to storage tissues after re-feeding. This transition is mediated by the rapid upregulation of ANGPTL8 expression in the liver and WAT, combined with a decrease in ANGPTL4 expression in WAT (51). The resultant secretion of a hepatic ANGPTL3-ANGPTL8 complex mediates endocrine inhibition of LPL in oxidative tissues. The increased synthesis of ANGPTL8 may attenuate LPL inhibition by ANGPTL4 in an autocrine/paracrine manner that favors TRLs processing in WAT.
LPL as a ligand
LPL improves the binding of CMs, β-VLDL, and apolipoprotein E (apoE)-containing liposomes to LDL receptor-related protein (LRP) (18). The pre-heparin LPL mass aids in the clearance of residual lipoproteins. LPL can act as a ligand for LRP and may mediate remnant uptake (54). Inactive LPL does not promote remnant uptake into Hep G2 according to research on denatured bovine milk LPL (55); LRP is the receptor for activated α2-macroglobulin (19,56,57). Eisenberg suggested that LPL primarily influences the binding of human plasma lipoproteins to heparan sulfate on cell surfaces and in the extracellular matrix (20). LPL binds to both the α2-macroglobulin receptor (α2MR)/LRP and β-VLDL. Dimeric LPL mediates the binding of β-VLDL to the receptor protein. LPL in combination with β-VLDL improves binding to α2MR/LRP. LPL-mediated binding and uptake of remnant particles induce the physiological remnant removal and pathophysiology of atherosclerosis (21). Catalytically inactive LPL mediates organ uptake of VLDL particles and selective uptake of cholesteryl ester into cells, resulting in lower VLDL triglyceride levels and myopathy (22).
GPIHBP1 (glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1)
GPIHBP1, a capillary endothelial cell GPI-anchored protein (11,25,40), is a dedicated LPL chaperone. GPIHBP1 transports LPL from the subendothelial spaces into the capillary lumen (11,24,41,58). LPL-mediated intravascular triglyceride processing is dependent on GPIHBP1-chaperoned LPL transport across capillaries (11,24,42). GPIHBP1 maintains LPL’s structure and catalytic activity (11,24,43,45). The literature suggests that GPIHBP1 chaperones LPL in four ways (11). First, LPL capture from subendothelial spaces is dependent on the GPIHBP1 protein found on the abluminal surface of capillary endothelial cells (41,44). Second, the binding of GPIHBP1 to LPL stabilizes its structure and activity (43-45). Third, GPIHBP1 transports LPL across endothelial cells in the capillary lumen to its site of action (41). Fourth, GPIHBP1-bound LPL is required for lipoprotein regulation in the bloodstream (42), allowing LPL-mediated lipoprotein processing to occur. We recently reported hypertriglyceridemia caused by GPIHBP1 autoantibodies (59). The discovery of inhibitory GPIHBP1 autoantibodies revealed a new etiology of acquired hypertriglyceridemia in some patients with no known mutations in LPL, GPIHBP1, APOC2, APOA5, or LMF1 (59-61).
Circulating levels of LPL and GPIHBP1
LPL is released into the bloodstream by heparin injection
LPL is released into the bloodstream after being detached from vascular endothelial cells by heparin injection (62). Although pre-heparin plasma contains a significantly large amount of LPL, the activity of TG hydrolysis is very low or non-detectable (23). Therefore, LPL in various lipoprotein disorders has been studied using post-heparin plasma (with intravascular heparin injection) (63-65). However, at room temperature, LPL activity in post-heparin plasma rapidly decreases at room temperature (7), making it unsuitable for routine clinical use. Because of the requirement for heparin injection, LPL determination has not been used in general clinical research. Heparin injection can cause bleeding, which is dangerous for patients with peptic ulcers or proliferative diabetic retinopathy. There are also issues with post-heparin LPL mass determination that prevent it from becoming a widely used test (66). An enzyme-linked immunosorbent assay was developed to detect LPL in human plasma using specific monoclonal antibodies (67,68). LPL concentration and activity measurements in post-heparin plasma have been used in clinical trials to detect LPL deficiency (69) but not to diagnose lipid disorders or the risk of cardiovascular disease. Because heparin injection causes LPL to dissociate from vascular endothelial cells, the measured concentration is not indicative of normal or pathological LPL levels in the bloodstream (70). Therefore, the importance of determining circulating LPL in the absence of heparin treatment, such as pre-heparin serum/plasma, should be considered.
The LPL mass and activity in pre-heparin and post-heparin plasma
The function, turnover, and transport of plasma LPL before and after heparin treatment differ significantly, as evidenced by LPL mass and activity. All of the parameters had a significant but distinct relationship with plasma lipoprotein lipid concentrations (17). The low correlation between pre- and post-heparin LPL may be due to pre-existing LPL (pre-heparin LPL) in post-heparin plasma (68). The percentage of LPL that separates from the entire vascular endothelial cell surface after heparin injection is unknown. It is clear that post-heparin LPL mass contains an artificial factor given that it is affected by variables such as heparin dose, the time elapsed after injection, and circulation (17,68). Pre-heparin LPL mass may indicate whole-body LPL activity because LPL hydrolyzes triglycerides, lowering serum triglyceride levels and increasing HDL-C (68). The serum pre-heparin LPL concentration is high enough to be measured. A comparative analysis reveals that post-heparin plasma LPL activity can replace pre-heparin serum LPL concentration (23). Therefore, using an automated LPL assay to measure the LPL concentration in pre-heparin serum can provide practical clinical applications in TG-rich patients without the need for heparin injection (68).
Pre- and post-heparin plasma LPL in TRLs metabolism
TRL-associated atherogenic dyslipidemia is characterized by elevated fasting triglycerides, remnant lipoproteins (RLPs), LDL-C levels, and small dense LDL cholesterol (sdLDL-C), as well as postprandial accumulation of TRLs (71). Post- and pre-heparin plasma LPL primarily metabolizes RLPs. LPL activity and concentration correlated inversely with RLP particle size as measured by the RLP-TG/RLP-C ratio in both pre-and post-heparin plasma. RLP particle size is consistent with pre-heparin plasma LPL concentration and post-heparin plasma LPL activity (23,72-77) (Table 2). Furthermore, both postprandial pre-heparin plasma LPL concentration and post-heparin plasma LPL activity were similarly inversely related to RLP particle size. Fasting post-heparin plasma LPL activity and postprandial pre-heparin plasma LPL concentration had the greatest similarity. Despite the inverse relationship between LPL concentration and RLP particle size (23,72-75,77), an increase in LPL is associated with an increase in RLPs (23,72-75,77). This suggests that insufficient hydrolysis of TG-rich lipoproteins by LPL on the endothelium after a fatty meal may result in RLP with a large particle size. RLPs are sdLDL-C precursors. Plasma sdLDL-C concentration is positively correlated with TG and RLPs but negatively correlated with LPL activity (23,72-74,76). Post-heparin plasma LPL activity and concentration correlated negatively with pre-heparin plasma TG, RLP-C, RLP-TG, and sdLDL-C concentrations (23,72-75,77). LPL concentration in pre-heparin plasma is more physiologically associated with adiponectin than maximum LPL activity or concentration in post-heparin plasma (Table 2). Therefore, LPL activity or concentration measured in post-heparin plasma may not accurately reflect the physiological state of TRL metabolism. This implies that pre-heparin plasma LPL activity may be more useful for diagnosis than measuring post-heparin plasma LPL concentration. The circulating levels of LPL are inversely related to TG, RLP-C, RLP-TG, and sdLDL-C (23,72-75,77) (Table 2).
Table 2
| Items | Association with pre-heparin LPL | Reference number |
|---|---|---|
| Metabolic parameters | ||
| Body weight | Inverse association | (78,79) |
| Fasting plasma glucose | Inverse association | (78,79) |
| Fasting plasma insulin | Inverse association | (78,79) |
| HOMA-IR | Inverse association | (78-81) |
| Triglyceride | Inverse association | (23,72-75,77,78,82,83) |
| Remnant lipoprotein | Inverse association | (23,72-75,77,78,82,83) |
| Small dense LDL-C | Inverse association | (23,72-75,77,78,82,83) |
| HDL-C | Positive association | (72,78) |
| Adiponectin | Positive association | (72,78) |
| GPIHBP1 | Positive association | (82) |
| Skeletal muscle | Positive association | (83) |
| Arteriosclerotic disease | ||
| Type 2 diabetes mellitus | Low pre-heparin LPL mass | (84-94) |
| Cardiovascular disease | Low pre-heparin LPL mass | (17,82,95-101) |
| Number of symptoms of metabolic syndrome | The higher the number of symptoms, the lower the pre-heparin LPL | (78,80) |
| Therapeutic approach | ||
| 5-hydroxytrptamine2A receptor antagonist | Increase pre-heparin LPL | (102) |
| Angiotensin II receptor antagonist | Increase pre-heparin LPL | (103) |
| Bezafibrate | Increase pre-heparin LPL | (104-106) |
| Colestimide | Decrease pre-heparin LPL | (107) |
| Incretin | Increase pre-heparin LPL | (6,108-112) |
| Insulin | Increase pre-heparin LPL | (84-87,91) |
| Metformin | Increase pre-heparin LPL | (113) |
| Pioglitazone/Troglitazone | Increase pre-heparin LPL | (113-118) |
| Statin | Increase pre-heparin LPL | (119-123) |
| No effect | (93,124,125) | |
| Decrease pre-heparin LPL | (93,126) | |
| LSG | Increase pre-heparin LPL | (127) |
| Konjac glucomannan | Increase pre-heparin LPL | (128) |
HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; GPIHBP1, glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1; LSG, laparoscopic sleeve gastrectomy; LPL, lipoprotein lipase.
LPL and adiponectin
Adiponectin, a fat-derived adipocytokine, has been linked to insulin resistance (129,130), lipoprotein metabolism, and abdominal fat (129). Insulin regulates LPL expression and production in adipocytes (131,132). Pre-heparin LPL has a strong relationship with plasma adiponectin (72,78) (Table 2). Both adiponectin and pre-heparin LPL levels fall as symptoms of metabolic diseases worsen (78,80), and they are inversely related to body weight and TG but positively related to HDL-C (78) (Table 2). Low LPL mass in pre-heparin serum indicates rising insulin resistance and adipose tissue accumulation (78) (Table 2). Plasma adiponectin and RLPs are inversely related (81,130,133). Reduced adiponectin levels are frequently associated with increased RLP levels in patients with high insulin resistance (129,133). VLDL and RLP hydrolysis is delayed by reduced LPL levels linked with low adiponectin levels (72,81,129,133). The mechanism that increases LPL activity in cardiomyocytes is well characterized (84,134). Adiponectin increases cell-surface expression and LPL activity time-dependently in adult rat cardiomyocytes (84). Adiponectin aids LPL activation by translocating it to the cell surface (84). Adiponectin increases fatty acid uptake in cardiomyocytes (134-137). Diabetic cardiomyopathy is defined by an increased dependence on free fatty acids for energy production in the myocardium and decreased glucose utilization (138). Increased cardiac LPL activity caused by adiponectin may be critical in the progression of heart failure. Decreased adiponectin levels linked to lower cardiac LPL raise plasma triglyceride concentrations. Cardiac-specific deletion of LPL is linked to heart dysfunction (139). Adiponectin increases insulin signaling and restores insulin sensitivity by reducing ectopic lipid storage in the liver and skeletal muscle. Adiponectin mediates these effects by stimulating LPL in increased muscle fat oxidation (140).
LPL and insulin sensitivity
Insulin resistance is closely linked to the development of atherosclerosis. In adipose tissue, insulin regulates LPL production (81,133). The biosynthesis of LPL is activated by an insulin-sensitive element in the LPL gene (133). LPL expression is increased in skeletal muscle and adipose tissue in response to insulin (81,133). Pre-heparin LPL mass reflects the total body LPL production and is linked to insulin resistance (141). Insulin resistance, measured by the homeostasis model assessment of insulin resistance (HOMA-IR) index, is considerably associated with pre-heparin serum LPL but not with post-heparin plasma LPL (81,133) (Table 2). Pre-heparin LPL mass correlates negatively with body weight, fasting blood glucose, HbA1c, fasting immunoreactive insulin (IRI), and HOMA-IR (78,79) (Table 2). Hypertriglyceridemia, high sdLDL-C, and low HDL-C are linked to insulin resistance (85-88,142) and may cause a decrease in LPL production (142). The degree of insulin resistance in metabolic syndrome may be linked to the pre-heparin LPL mass (which reflects insulin sensitivity) and oxidative stress (78).
LPL and diabetes
Insulin plays a major role in regulating pre-heparin LPL mass. Patients with type 2 diabetes mellitus have significantly lower LPL production and circulating pre-heparin LPL mass than non-diabetic healthy controls (84-94) (Table 2). Pre-heparin LPL mass correlates negatively with HbA1c in patients with diabetes (92) (Table 2). Post-heparin LPL activity reportedly declines in diabetes (90). Pre-heparin LPL mass and HDL-C levels are significantly increased by insulin injection, followed by a drop in FBS (84-87,91) (Table 2). LPL activity in adipose tissue is significantly lowered in diabetic men but not in diabetic women (92). Decreased LPL lipolysis of plasma TG-rich lipoproteins may cause the inferior lipid profile found in men with poorly controlled type 2 diabetes than women (93). LPL activity in adipose tissue is significantly reduced in men with diabetes but not in women (92). In type 2 diabetes mellitus, low adiponectin in plasma is linked to low post-heparin LPL (94). Pre-heparin LPL mass indirectly shows the amount of working LPL activity in vivo (89).
LPL and fatty acid metabolism in the diabetic heart
The regulatory mechanism of LPL in the heart was thoroughly evaluated and well-illustrated by Rodrigues and colleagues (143). On the apical side of coronary endothelial cells, GPIHBP1-bound LPL hydrolyzes triglycerides, synthesizes fatty acids, and supplies them to the cardiomyocyte (143,144). In cardiomyocytes, fatty acids undergo mitochondrial b-oxidation and oxidative phosphorylation to generate ATP or accumulate as lipid metabolites/droplets (143). Accumulated lipid intermediates activate insulin signaling and substrate utilization (143). In the heart, 95% of the generated ATP is acquired from glucose and FAs through mitochondrial metabolism (143). The heart cannot synthesize FAs and obtains them from other sources (143). LPL-mediated lipolysis of lipoproteins is a critical source of FAs in the heart (145). In type 2 diabetes mellitus, glucose utilization efficiency declines due to increased insulin resistance and insufficient insulin action. Following diabetes, the heart shifts its primary energy source from glucose to fatty acids, causing diabetic cardiomyopathy (143-145). In diabetes, increased fatty acid use due to underutilization of glucose is compensated by an increase in vascular LPL or adipose tissue lipolysis. There is a mismatch between the delivery of FAs and their oxidation in the diabetic heart, causing lipid metabolite accumulation and myocyte LD synthesis (143). This mediates lipid-induced insulin resistance, cell death, and, eventually, diabetic cardiomyopathy (67-69,87,88,143-145).
LPL and coronary heart diseases
Pre-heparin LPL levels and LPL activity are decreased in cardiovascular disease, including plaque instability, coronary stenosis, coronary vasospasm, and acute myocardial infarction, as repeatedly highlighted in this review (17,82,95-101) (Table 2). Shirai and colleagues reported that pre-heparin LPL mass was the highest risk factor for coronary stenosis than other risk factors such as age, smoking, family history, hypertension, hyperuricemia, diabetes mellitus, total cholesterol, triglyceride, HDL-C, and BMI (95-97,104) (Table 2). The hepatic triglyceride lipase (HTGL) concentration demonstrates positive correlations, while GPIHBP1 shows inverse correlations with RLP-C and sdLDL-C. Elevated HTGL is linked to an increased risk of CAD, while increased LPL is associated with a reduced risk of cardiovascular disease (82) (Table 2). Low LPL production was found to be associated with atherosclerosis and the overexpression of LPL decreased serum TRL, particularly RLPs, in mice (146). Low pre-heparin LPL, hypertriglyceridemia, and higher sdLDL are independent risk factors for cardiovascular diseases and are considerably related to each other (17,95-100). Furthermore, a prospective study revealed that low pre-and/or post-heparin LPL mass predicts future coronary events (147).
LPL and exercise
Pre-heparin LPL and GPIHBP1 serum concentrations assessed in young Japanese men were shown to be significantly high in skeletal muscle-rich participants and positively correlated with skeletal muscle mass. Increasing skeletal muscle mass increases energy use by boosting TRL hydrolysis through circulating LPL and GPIHBP1 concentrations. In contrast, elevated HTGL serum concentrations are linked to a rise in serum LDL-C synthesis that is independent of skeletal muscle mass (83). Post-heparin plasma LPL activity increases after prolonged exercise (148). Increased post-heparin LPL activity was observed to be significantly correlated with exercise-induced reductions in fasting and postprandial triacylglycerol (TAG) concentrations (149). Skeletal muscle LPL activity is maximized more than 8 h after exercise (150). On the contrary, moderate-intensity cycling performed the day before loading moderate-fat food reduced postprandial serum TAG concentrations in young men without affecting pre-heparin LPL concentrations measured in the fasted and postprandial states the following day (151). Further study is required to determine the effect of exercise on circulating pre-heparin LPL levels. Because there are distinctions between men and women in body composition, such as body fat percentage and muscle mass, gender differences are anticipated in the effect of exercise on circulating pre-heparin LPL levels.
LPL and lipid-lowering therapy
Plasma triglyceride levels are more than just a marker. It is a risk factor for coronary artery disease (152,153) and one of the risks associated with statin therapy (154). In the future, lipid-directed treatment will include treating TRL in specific patient populations and lowering LDL-C levels (6). LPL plays an important role in TRL hydrolysis. The fasting and postprandial blood triglyceride levels are determined by LPL-mediated lipolysis and hepatic uptake of remnant particles (6,155). Reduced plasma LPL mass is associated with an increased risk of coronary artery disease (95,97) (Table 2). The administration of drugs such as fibrate, insulin sensitizers, and statins to healthy volunteers or patients with diseases that are likely to progress arteriosclerosis affects the plasma LPL mass concentration. Triglyceride levels were lower after taking bezafibrate, which is thought to be due in part to increased LPL production (156,157). Bezafibrate administration increased LPL mass and activity in pre- and post-heparin plasma (104-106) (Table 2). Insulin sensitizer administration activates PPARγ, such as pioglitazone and troglitazone, and increases LPL mass and activity (113-118) (Table 2). Metformin raises pre-heparin LPL levels (113) (Table 2). Glucagon-like peptide 1 (GIP), one of the incretins, inhibits CM secretion (6,108,109) and activates LPL (110-112) (Table 2). Recently, adding konjac glucomannan (KGM) powder to rice gruel reduced TG while increasing LPL and GPIHBP1 (128) (Table 2). It is unknown what mechanism increased LPL in response to KGM supplementation in rice gruel (128). The LPL increase could be explained by incretin induction, which promotes KGM intestinal activity. Statins’ effects on LPL mass and activity are contradictory. The effect of statin administration includes: (I) increased LPL mass or activity (112,113,119-123); (II) had no effect (93,124,125); (III) decreased LPL mass or activity (93,126) (Table 2). Colestimide, but not ezetimibe, considerably reduced plasma LPL mass (107) (Table 2). In addition to these human studies, statins also stimulate LPL synthesis in vitro studies. Statin promoted LPL expression in preadipocytes (158) and skeletal muscle cells (159). Studies on the effects of statins revealed no clear relationship between changes in lipase mass and changes in plasma lipid levels (97). Additionally, angiotensin II receptor antagonist (103) and 5-hydroxytryptamine2A receptor antagonist (102) are also known to increase serum LPL mass. LPL activity-related genetic abnormalities mediate cardiovascular risk. Loss-of-function mutations in apoC3, for example, which is an LPL inhibitor, decrease the risk of coronary artery disease (152). In contrast, loss-of-function mutations in apoA5, which is an LPL activator, increase the risk of coronary artery disease (160). Furthermore, a surgical method also attenuated pre-heparin LPL. Pre-heparin LPL levels increased during BW reduction and laparoscopic sleeve gastrectomy (LSG), a bariatric surgical procedure in obese patients (127) (Table 2). LSG effectively improves diabetes, hypertension, and dyslipidemia (161,162). Bariatric surgery, including LSG, has amazing therapeutic effects for obesity and obesity-related diseases (160-162). During coronary angiography, LPL increased 15 minutes after heparin administration, and TG and sdLDL decreased, but returned to the basal levels 4 hours later (82). In hemodialysis, administration of heparin transiently increases LPL and decreases TG. After that, LPL and TG return to pre-heparin levels. Repeated administration of heparin in hemodialysis depletes LPL stores, therefore, chronic dialysis patients have decreased LPL activity, dyslipidemia, and an increased risk of CVD (163,164). At present, administration of heparin for the treatment of hypertriglyceridemia due to increased LPL has not been investigated.
Conclusions
Despite optimal statin treatment, the risk of cardiovascular disease persists. Reducing the prevalence of cardiovascular diseases is critical for reducing the number of NCD patients. Epidemiological and genomic research suggests the contribution of TRLs in the development of cardiovascular diseases. According to natural selection studies, novel triglyceride-lowering therapies can reduce cardiovascular risk. Clinical trials are currently underway to determine the efficacy of LPL activity modulators that inhibit apoC3 or ANGPTL3. The clinical significance of pre-heparin LPL measurement must be determined to assess the efficacy of these drugs. LPL activity is highly regulated at the transcriptional, post-transcriptional, translational, and post-translational levels. We have successfully developed assay systems for human LPL and GPIHBP1, as well as mouse assay systems. Using these measurement systems should lead to a better understanding of the clinical significance of pre-heparin LPL and GPIHBP1.
Acknowledgments
We thank Mayumi Nishiyama (Department of Clinical Laboratory Medicine, Gunma University Graduate School of Medicine, Maebashi, Japan) for their technical assistance and helpful discussion.
Funding: This work was supported in part by Grants-in-Aid 20K07841 and 23K08003 (to TK) for scientific re-search from the Ministry of Education, Culture, Sports Science, and Technology of Japan.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tatsuo Shimosawa) for the series “New Biomarkers in Non-communicable Diseases” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-12/rc
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-12/coif). The series “New Biomarkers in Non-communicable Diseases” was commissioned by the editorial office without any funding or sponsorship. TK was supported by Grants-in-Aid 20K07841 and 23K08003 for scientific research from the Ministry of Education, Culture, Sports Science, and Technology of Japan. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Geneva: Noncommunicable diseases; 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- Rosenson RS, Shaik A, Song W. New Therapies for Lowering Triglyceride-Rich Lipoproteins: JACC Focus Seminar 3/4. J Am Coll Cardiol 2021;78:1817-30. [Crossref] [PubMed]
- Shaik A, Rosenson RS. Genetics of Triglyceride-Rich Lipoproteins Guide Identification of Pharmacotherapy for Cardiovascular Risk Reduction. Cardiovasc Drugs Ther 2021;35:677-90. [Crossref] [PubMed]
- Miller M. Is triglyceride therapy worth the effort? Cleve Clin J Med 2015;82:162-6. [Crossref] [PubMed]
- Kim K, Ginsberg HN, Choi SH. New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins. Diabetes Metab J 2022;46:817-8. [Crossref] [PubMed]
- Laufs U, Parhofer KG, Ginsberg HN, et al. Clinical review on triglycerides. Eur Heart J 2020;41:99-109c. [Crossref] [PubMed]
- Nilsson-Ehle P, Garfinkel AS, Schotz MC. Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem 1980;49:667-93. [Crossref] [PubMed]
- Hahn PF. Abolishment of alimentary lipemia following injection of heparin. Science 1943;98:19-20. [Crossref] [PubMed]
- Korn ED. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem 1955;215:1-14. [Crossref] [PubMed]
- Korn ED. Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J Biol Chem 1955;215:15-26. [Crossref] [PubMed]
- Young SG, Fong LG, Beigneux AP, et al. GPIHBP1 and Lipoprotein Lipase, Partners in Plasma Triglyceride Metabolism. Cell Metab 2019;30:51-65. [Crossref] [PubMed]
- Basu D, Goldberg IJ. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Curr Opin Lipidol 2020;31:154-60. [Crossref] [PubMed]
- Braun JE, Severson DL. Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem J 1992;287:337-47. [Crossref] [PubMed]
- Saxena U, Klein MG, Goldberg IJ. Transport of lipoprotein lipase across endothelial cells. Proc Natl Acad Sci U S A 1991;88:2254-8. [Crossref] [PubMed]
- Li Y, He PP, Zhang DW, et al. Lipoprotein lipase: from gene to atherosclerosis. Atherosclerosis 2014;237:597-608. [Crossref] [PubMed]
- Peterson J, Bihain BE, Bengtsson-Olivecrona G, et al. Fatty acid control of lipoprotein lipase: a link between energy metabolism and lipid transport. Proc Natl Acad Sci U S A 1990;87:909-13. [Crossref] [PubMed]
- Tornvall P, Olivecrona G, Karpe F, et al. Lipoprotein lipase mass and activity in plasma and their increase after heparin are separate parameters with different relations to plasma lipoproteins. Arterioscler Thromb Vasc Biol 1995;15:1086-93. [Crossref] [PubMed]
- Beisiegel U, Weber W, Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A 1991;88:8342-6. [Crossref] [PubMed]
- Chappell DA, Fry GL, Waknitz MA, et al. The low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor binds and mediates catabolism of bovine milk lipoprotein lipase. J Biol Chem 1992;267:25764-7. [Crossref] [PubMed]
- Eisenberg S, Sehayek E, Olivecrona T, et al. Lipoprotein lipase enhances binding of lipoproteins to heparan sulfate on cell surfaces and extracellular matrix. J Clin Invest 1992;90:2013-21. [Crossref] [PubMed]
- Nykjaer A, Bengtsson-Olivecrona G, Lookene A, et al. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds lipoprotein lipase and beta-migrating very low density lipoprotein associated with the lipase. J Biol Chem 1993;268:15048-55. [Crossref] [PubMed]
- Merkel M, Kako Y, Radner H, et al. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo. Proc Natl Acad Sci U S A 1998;95:13841-6. [Crossref] [PubMed]
- Shirakawa T, Nakajima K, Shimomura Y, et al. Comparison of the effect of post-heparin and pre-heparin lipoprotein lipase and hepatic triglyceride lipase on remnant lipoprotein metabolism. Clin Chim Acta 2015;440:193-200. [Crossref] [PubMed]
- Kristensen KK, Leth-Espensen KZ, Kumari A, et al. GPIHBP1 and ANGPTL4 Utilize Protein Disorder to Orchestrate Order in Plasma Triglyceride Metabolism and Regulate Compartmentalization of LPL Activity. Front Cell Dev Biol 2021;9:702508. [Crossref] [PubMed]
- Wong H, Schotz MC. The lipase gene family. J Lipid Res 2002;43:993-9. [Crossref] [PubMed]
- Rosenson RS, Davidson MH, Hirsh BJ, et al. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol 2014;64:2525-40. [Crossref] [PubMed]
- Berbée JF, van der Hoogt CC, Sundararaman D, et al. Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL. J Lipid Res 2005;46:297-306. [Crossref] [PubMed]
- Larsson M, Vorrsjö E, Talmud P, et al. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem 2013;288:33997-4008. [Crossref] [PubMed]
- Kinnunen PK, Jackson RL, Smith LC, et al. Activation of lipoprotein lipase by native and synthetic fragments of human plasma apolipoprotein C-II. Proc Natl Acad Sci U S A 1977;74:4848-51. [Crossref] [PubMed]
- Ginsberg HN, Le NA, Goldberg IJ, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest 1986;78:1287-95. [Crossref] [PubMed]
- Grosskopf I, Baroukh N, Lee SJ, et al. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler Thromb Vasc Biol 2005;25:2573-9. [Crossref] [PubMed]
- Shimizugawa T, Ono M, Shimamura M, et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem 2002;277:33742-8. [Crossref] [PubMed]
- Köster A, Chao YB, Mosior M, et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 2005;146:4943-50. [Crossref] [PubMed]
- Sukonina V, Lookene A, Olivecrona T, et al. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci U S A 2006;103:17450-5. [Crossref] [PubMed]
- Quagliarini F, Wang Y, Kozlitina J, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci U S A 2012;109:19751-6. [Crossref] [PubMed]
- Beigneux AP, Allan CM, Sandoval NP, et al. Lipoprotein lipase is active as a monomer. Proc Natl Acad Sci U S A 2019;116:6319-28. [Crossref] [PubMed]
- Kristensen KK, Leth-Espensen KZ, Mertens HDT, et al. Unfolding of monomeric lipoprotein lipase by ANGPTL4: Insight into the regulation of plasma triglyceride metabolism. Proc Natl Acad Sci U S A 2020;117:4337-46. [Crossref] [PubMed]
- Leth-Espensen KZ, Kristensen KK, Kumari A, et al. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc Natl Acad Sci U S A 2021;118:e2026650118. [Crossref] [PubMed]
- Sundberg EL, Deng Y, Burd CG. Syndecan-1 Mediates Sorting of Soluble Lipoprotein Lipase with Sphingomyelin-Rich Membrane in the Golgi Apparatus. Dev Cell 2019;51:387-398.e4. [Crossref] [PubMed]
- Beigneux AP, Davies BS, Gin P, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 2007;5:279-91. [Crossref] [PubMed]
- Davies BS, Beigneux AP, Barnes RH 2nd, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab 2010;12:42-52. [Crossref] [PubMed]
- Goulbourne CN, Gin P, Tatar A, et al. The GPIHBP1-LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab 2014;19:849-60. [Crossref] [PubMed]
- Mysling S, Kristensen KK, Larsson M, et al. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. Elife 2016;5:e12095. [Crossref] [PubMed]
- Kristensen KK, Midtgaard SR, Mysling S, et al. A disordered acidic domain in GPIHBP1 harboring a sulfated tyrosine regulates lipoprotein lipase. Proc Natl Acad Sci U S A 2018;115:E6020-9. [Crossref] [PubMed]
- Mysling S, Kristensen KK, Larsson M, et al. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. Elife 2016;5:e20958. [Crossref] [PubMed]
- Chi X, Britt EC, Shows HW, et al. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab 2017;6:1137-49. [Crossref] [PubMed]
- Chen YQ, Pottanat TG, Siegel RW, et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res 2020;61:1203-20. [Crossref] [PubMed]
- Gusarova V, Banfi S, Alexa-Braun CA, et al. ANGPTL8 Blockade With a Monoclonal Antibody Promotes Triglyceride Clearance, Energy Expenditure, and Weight Loss in Mice. Endocrinology 2017;158:1252-9. [Crossref] [PubMed]
- Haller JF, Mintah IJ, Shihanian LM, et al. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res 2017;58:1166-73. [Crossref] [PubMed]
- Kovrov O, Kristensen KK, Larsson E, et al. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. J Lipid Res 2019;60:783-93. [Crossref] [PubMed]
- Oldoni F, Cheng H, Banfi S, et al. ANGPTL8 has both endocrine and autocrine effects on substrate utilization. JCI Insight 2020;5:e138777. [Crossref] [PubMed]
- Cushing EM, Chi X, Sylvers KL, et al. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol Metab 2017;6:809-18. [Crossref] [PubMed]
- Zhang R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol 2016;6:150272. [Crossref] [PubMed]
- Beisiegel U, Krapp A, Weber W, et al. The role of lipases and LRP in the catabolism of triglyceride-rich lipoproteins. Z Gastroenterol 1996;34:108-9. [PubMed]
- Huff MW, Miller DB, Wolfe BM, et al. Uptake of hypertriglyceridemic very low density lipoproteins and their remnants by HepG2 cells: the role of lipoprotein lipase, hepatic triglyceride lipase, and cell surface proteoglycans. J Lipid Res 1997;38:1318-33. [Crossref] [PubMed]
- Strickland DK, Ashcom JD, Williams S, et al. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem 1990;265:17401-4. [Crossref] [PubMed]
- Kristensen T, Moestrup SK, Gliemann J, et al. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Lett 1990;276:151-5. [Crossref] [PubMed]
- Davies BS, Goulbourne CN, Barnes RH 2nd, et al. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J Lipid Res 2012;53:2690-7. [Crossref] [PubMed]
- Beigneux AP, Miyashita K, Ploug M, et al. Autoantibodies against GPIHBP1 as a Cause of Hypertriglyceridemia. N Engl J Med 2017;376:1647-58. [Crossref] [PubMed]
- Lutz J, Dunaj-Kazmierowska M, Arcan S, et al. Chylomicronemia From GPIHBP1 Autoantibodies Successfully Treated With Rituximab: A Case Report. Ann Intern Med 2020;173:764-5. [Crossref] [PubMed]
- Miyashita K, Lutz J, Hudgins LC, et al. Chylomicronemia from GPIHBP1 autoantibodies. J Lipid Res 2020;61:1365-76. [Crossref] [PubMed]
- Vilella E, Joven J, Fernández M, et al. Lipoprotein lipase in human plasma is mainly inactive and associated with cholesterol-rich lipoproteins. J Lipid Res 1993;34:1555-64. [Crossref] [PubMed]
- Huttunen JK, Ehnholm C, Kekki M, et al. Post-heparin plasma lipoprotein lipase and hepatic lipase in normal subjects and in patients with hypertriglyceridaemia: correlations to sex, age and various parameters of triglyceride metabolism. Clin Sci Mol Med 1976;50:249-60. [Crossref] [PubMed]
- Taskinen MR, Nikkilä EA, Kuusi T. Lipoprotein lipase activity of adipose tissue, skeletal muscle and post-heparin plasma in primary endogenous hypertriglyceridaemia: relation to lipoprotein pattern and to obesity. Eur J Clin Invest 1982;12:433-8. [Crossref] [PubMed]
- Taskinen MR, Kuusi T. Enzymes involved in triglyceride hydrolysis. Baillieres Clin Endocrinol Metab 1987;1:639-66. [Crossref] [PubMed]
- Hirano T, Nishioka F, Murakami T. Measurement of the serum lipoprotein lipase concentration is useful for studying triglyceride metabolism: Comparison with postheparin plasma. Metabolism 2004;53:526-31. [Crossref] [PubMed]
- Peterson J, Fujimoto WY, Brunzell JD. Human lipoprotein lipase: relationship of activity, heparin affinity, and conformation as studied with monoclonal antibodies. J Lipid Res 1992;33:1165-70. [Crossref] [PubMed]
- Ikeda Y, Takagi A, Ohkaru Y, et al. A sandwich-enzyme immunoassay for the quantification of lipoprotein lipase and hepatic triglyceride lipase in human postheparin plasma using monoclonal antibodies to the corresponding enzymes. J Lipid Res 1990;31:1911-24. [Crossref] [PubMed]
- Brunzell JD, Deeb SS. Familial lipoprotein lipase deficiency, apo C-II deficiency and hepatic lipase deficiency. In: Scriver CR, Beaudet AL, Sly WS, et al. editors. The metabolic and molecular basis of inherited disease. New York: Mc Graw-Hill Inc.; 2001:2789-816.
- Machida T, Miyashita K, Sone T, et al. Determination of serum lipoprotein lipase using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta 2015;442:130-5. [Crossref] [PubMed]
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640-5. [Crossref] [PubMed]
- Shirakawa T, Nakajima K, Yatsuzuka S, et al. The role of circulating lipoprotein lipase and adiponectin on the particle size of remnant lipoproteins in patients with diabetes mellitus and metabolic syndrome. Clin Chim Acta 2015;440:123-32. [Crossref] [PubMed]
- Nakajima K, Kobayashi J, Mabuchi H, et al. Association of angiopoietin-like protein 3 with hepatic triglyceride lipase and lipoprotein lipase activities in human plasma. Ann Clin Biochem 2010;47:423-31. [Crossref] [PubMed]
- Nakajima K, Nakano T, Tokita Y, et al. The characteristics of remnant lipoproteins in the fasting and postprandial plasma. Clin Chim Acta 2012;413:1077-86. [Crossref] [PubMed]
- Nakajima K, Nagamine T, Fujita MQ, et al. Apolipoprotein B-48: a unique marker of chylomicron metabolism. Adv Clin Chem 2014;64:117-77. [Crossref] [PubMed]
- Deighan CJ, Caslake MJ, McConnell M, et al. The atherogenic lipoprotein phenotype: small dense LDL and lipoprotein remnants in nephrotic range proteinuria. Atherosclerosis 2001;157:211-20. [Crossref] [PubMed]
- Sato K, Okajima F, Miyashita K, et al. The majority of lipoprotein lipase in plasma is bound to remnant lipoproteins: A new definition of remnant lipoproteins. Clin Chim Acta 2016;461:114-25. [Crossref] [PubMed]
- Saiki A, Oyama T, Endo K, et al. Preheparin serum lipoprotein lipase mass might be a biomarker of metabolic syndrome. Diabetes Res Clin Pract 2007;76:93-101. [Crossref] [PubMed]
- Hanyu O, Miida T, Obayashi K, et al. Lipoprotein lipase (LPL) mass in preheparin serum reflects insulin sensitivity. Atherosclerosis 2004;174:385-90. [Crossref] [PubMed]
- Ryo M, Nakamura T, Kihara S, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J 2004;68:975-81. [Crossref] [PubMed]
- Maruyoshi H, Kojima S, Funahashi T, et al. Adiponectin is inversely related to plasminogen activator inhibitor type 1 in patients with stable exertional angina. Thromb Haemost 2004;91:1026-30. [Crossref] [PubMed]
- Muraba Y, Koga T, Shimomura Y, et al. The role of plasma lipoprotein lipase, hepatic lipase and GPIHBP1 in the metabolism of remnant lipoproteins and small dense LDL in patients with coronary artery disease. Clin Chim Acta 2018;476:146-53. [Crossref] [PubMed]
- Matsumoto R, Tsunekawa K, Shoho Y, et al. Association between skeletal muscle mass and serum concentrations of lipoprotein lipase, GPIHBP1, and hepatic triglyceride lipase in young Japanese men. Lipids Health Dis 2019;18:84. [Crossref] [PubMed]
- Ganguly R, Schram K, Fang X, et al. Adiponectin increases LPL activity via RhoA/ROCK-mediated actin remodelling in adult rat cardiomyocytes. Endocrinology 2011;152:247-54. [Crossref] [PubMed]
- Feingold KR, Grunfeld C, Pang M, et al. LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler Thromb 1992;12:1496-502. [Crossref] [PubMed]
- Reaven GM. Role of insulin resistance in human disease. Diabetes 1988;37:1595-607. [Crossref] [PubMed]
- Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med 1992;231:25-30. [Crossref] [PubMed]
- Eliasson B, Mero N, Taskinen MR, et al. The insulin resistance syndrome and postprandial lipid intolerance in smokers. Atherosclerosis 1997;129:79-88. [Crossref] [PubMed]
- Miyashita Y, Shirai K, Itoh Y, et al. Low lipoprotein lipase mass in preheparin serum of type 2 diabetes mellitus patients and its recovery with insulin therapy. Diabetes Res Clin Pract 2002;56:181-7. [Crossref] [PubMed]
- Nikkilä EA, Huttunen JK, Ehnholm C. Postheparin plasma lipoprotein lipase and hepatic lipase in diabetes mellitus. Relationship to plasma triglyceride metabolism. Diabetes 1977;26:11-21. [Crossref] [PubMed]
- Maser RE, Lenhard MJ, Pohlig RT, et al. Pre-heparin lipoprotein lipase mass as a potential mediator in the association between adiponectin and HDL-cholesterol in type 2 diabetes. J Clin Transl Endocrinol 2016;7:7-11. [Crossref] [PubMed]
- Taskinen MR, Nikkilä EA, Kuusi T, et al. Lipoprotein lipase activity and serum lipoproteins in untreated type 2 (insulin-independent) diabetes associated with obesity. Diabetologia 1982;22:46-50. [Crossref] [PubMed]
- Kobayashi J, Maruyama T, Watanabe H, et al. Gender differences in the effect of type 2 diabetes on serum lipids, pre-heparin plasma lipoprotein lipase mass and other metabolic parameters in Japanese population. Diabetes Res Clin Pract 2003;62:39-45. [Crossref] [PubMed]
- von Eynatten M, Schneider JG, Humpert PM, et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia: an association independent of systemic inflammation and insulin resistance. Diabetes Care 2004;27:2925-9. [Crossref] [PubMed]
- Hitsumoto T, Ohsawa H, Uchi T, et al. Preheparin serum lipoprotein lipase mass is negatively related to coronary atherosclerosis. Atherosclerosis 2000;153:391-6. [Crossref] [PubMed]
- Hitsumoto T, Yoshinaga K, Noike H, et al. Clinical significance of preheparin serum lipoprotein lipase mass in coronary vasospasm. Jpn Circ J 2001;65:539-44. [Crossref] [PubMed]
- Hitsumoto T, Yoshinaga K, Aoyagi K, et al. Association between preheparin serum lipoprotein lipase mass and acute myocardial infarction in Japanese men. J Atheroscler Thromb 2002;9:163-9. [Crossref] [PubMed]
- Austin MA, Krauss RM. Genetic control of low-density-lipoprotein subclasses. Lancet 1986;2:592-5. [Crossref] [PubMed]
- Koba S, Hirano T. Small dense low-density lipoprotein in Japanese men with coronary artery disease. Ann Intern Med 2000;132:762. [Crossref] [PubMed]
- Austin MA, Breslow JL, Hennekens CH, et al. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988;260:1917-21. [Crossref] [PubMed]
- Kobayashi J, Nohara A, Kawashiri MA, et al. Serum lipoprotein lipase mass: clinical significance of its measurement. Clin Chim Acta 2007;378:7-12. [Crossref] [PubMed]
- Nagayama D, Ohira M, Saiki A, et al. Sarpogrelate hydrochloride decreases cardio-ankle vascular index accompanied by increased serum lipoprotein lipase mass in type 2 diabetic patients. Int Heart J 2014;55:337-41. [Crossref] [PubMed]
- Hitsumoto T, Takahashi M, Iizuka T, et al. Effect of the angiotensin II receptor antagonist telmisartan on lipoprotein lipase mass in preheparin serum. J Atheroscler Thromb 2008;15:138-45. [Crossref] [PubMed]
- Totsuka M, Miyashita Y, Ito Y, et al. Enhancement of preheparin serum lipoprotein lipase mass by bezafibrate administration. Atherosclerosis 2000;153:175-9. [Crossref] [PubMed]
- Kloss G, Behrandt J, Vollmar J, et al. Effect of bezafibrate on the activity of lipoprotein lipase and hepatic triglyceride hydrolase in healthy volunteers. In: Greten H, Lang PD, Schettler G. editors. Lipoproteins and coronary heart disease. New York: Witzstock Verlag; 1980:182-4.
- Kobayashi J, Takahashi K, Tashiro J, et al. Effects of treatment with bezafibrate on lipoprotein lipase activity and mass in patients with hypertriglyceridemia. Arzneimittelforschung 1994;44:145-8. [PubMed]
- Tada H, Kobayashi J, Kawashiri MA, et al. Changes in lipoprotein lipase and endothelial lipase mass in familial hypercholesterolemia during three-drug lipid-lowering combination therapy. Lipids Health Dis 2016;15:66. [Crossref] [PubMed]
- Xiao C, Dash S, Morgantini C, et al. Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: The Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol. Diabetes 2016;65:1767-78. [Crossref] [PubMed]
- Stahel P, Xiao C, Hegele RA, et al. The Atherogenic Dyslipidemia Complex and Novel Approaches to Cardiovascular Disease Prevention in Diabetes. Can J Cardiol 2018;34:595-604. [Crossref] [PubMed]
- Wilson JM, Nikooienejad A, Robins DA, et al. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes Metab 2020;22:2451-9. [Crossref] [PubMed]
- Kim SJ, Nian C, McIntosh CH. GIP increases human adipocyte LPL expression through CREB and TORC2-mediated trans-activation of the LPL gene. J Lipid Res 2010;51:3145-57. [Crossref] [PubMed]
- Kim SJ, Nian C, McIntosh CH. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J Biol Chem 2007;282:8557-67. [Crossref] [PubMed]
- Ohira M, Miyashita Y, Ebisuno M, et al. Effect of metformin on serum lipoprotein lipase mass levels and LDL particle size in type 2 diabetes mellitus patients. Diabetes Res Clin Pract 2007;78:34-41. [Crossref] [PubMed]
- Auwerx J, Schoonjans K, Fruchart JC, et al. Regulation of triglyceride metabolism by PPARs: fibrates and thiazolidinediones have distinct effects. J Atheroscler Thromb 1996;3:81-9. [Crossref] [PubMed]
- Shirai K, Itoh Y, Sasaki H, et al. The effect of insulin sensitizer, troglitazone, on lipoprotein lipase mass in preheparin serum. Diabetes Res Clin Pract 1999;46:35-41. [Crossref] [PubMed]
- Cavaghan MK, Ehrmann DA, Byrne MM, et al. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. J Clin Invest 1997;100:530-7. [Crossref] [PubMed]
- Suter SL, Nolan JJ, Wallace P, et al. Metabolic effects of new oral hypoglycemic agent CS-045 in NIDDM subjects. Diabetes Care 1992;15:193-203. [Crossref] [PubMed]
- Ohira M, Yamaguchi T, Saiki A, et al. Pioglitazone improves the cardio-ankle vascular index in patients with type 2 diabetes mellitus treated with metformin. Diabetes Metab Syndr Obes 2014;7:313-9. [Crossref] [PubMed]
- Endo K, Miyashita Y, Saiki A, et al. Atorvastatin and pravastatin elevated pre-heparin lipoprotein lipase mass of type 2 diabetes with hypercholesterolemia. J Atheroscler Thromb 2004;11:341-7. [Crossref] [PubMed]
- Verd JC, Peris C, Alegret M, et al. Different effect of simvastatin and atorvastatin on key enzymes involved in VLDL synthesis and catabolism in high fat/cholesterol fed rabbits. Br J Pharmacol 1999;127:1479-85. [Crossref] [PubMed]
- Sato A, Watanabe K, Fukuzumi H, et al. Effect of simvastatin (MK-733) on plasma triacylglycerol levels in rats. Biochem Pharmacol 1991;41:1163-72. [Crossref] [PubMed]
- Cabezas MC, de Bruin TW, Kock LA, et al. Simvastatin improves chylomicron remnant removal in familial combined hyperlipidemia without changing chylomicron conversion. Metabolism 1993;42:497-503. [Crossref] [PubMed]
- Nagayama D, Saiki A, Watanabe Y, et al. Prevention of Cardiovascular Events with Pitavastatin is Associated with Increased Serum Lipoprotein Lipase Mass Level: Subgroup Analysis of the TOHO-LIP. J Atheroscler Thromb 2022;29:451-63. [Crossref] [PubMed]
- Heller FR, Descamps OS, Hondekijin JC, et al. Atorvastatin and the plasma activities of lipoprotein lipase, hepatic lipase and lecithin:cholesterol acyltransferase in patients with mixed hyperlipidemia. Eur J Int Med 2000;11:33-8. [Crossref]
- Alegret M, Verd JC, Díaz C, et al. Effect of hypolipidemic drugs on key enzyme activities related to lipid metabolism in normolipidemic rabbits. Eur J Pharmacol 1998;347:283-91. [Crossref] [PubMed]
- Hoogerbrugge N, Jansen H. Atorvastatin increases low-density lipoprotein size and enhances high-density lipoprotein cholesterol concentration in male, but not in female patients with familial hypercholesterolemia. Atherosclerosis 1999;146:167-74. [Crossref] [PubMed]
- Ohira M, Yamaguchi T, Saiki A, et al. Laparoscopic Sleeve Gastrectomy Significantly Increases Serum Lipoprotein Lipase Level in Obese Patients. Obes Facts 2019;12:357-68. [Crossref] [PubMed]
- Nagasawa T, Kimura T, Yoshida A, et al. Konjac Glucomannan Attenuated Triglyceride Metabolism during Rice Gruel Tolerance Test. Nutrients 2021;13:2191. [Crossref] [PubMed]
- Chan DC, Watts GF, Ng TW, et al. Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism. Clin Chem 2005;51:578-85. [Crossref] [PubMed]
- Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941-6. [Crossref] [PubMed]
- Ong JM, Kirchgessner TG, Schotz MC, et al. Insulin increases the synthetic rate and messenger RNA level of lipoprotein lipase in isolated rat adipocytes. J Biol Chem 1988;263:12933-8. [Crossref] [PubMed]
- Semenkovich CF, Wims M, Noe L, et al. Insulin regulation of lipoprotein lipase activity in 3T3-L1 adipocytes is mediated at posttranscriptional and posttranslational levels. J Biol Chem 1989;264:9030-8. [Crossref] [PubMed]
- Yatsuzuka S, Shimomura Y, Akuzawa M, et al. Plasma adiponectin is a more specific marker of fatty liver than a marker of metabolic syndrome in Japanese men. Ann Clin Biochem 2014;51:68-79. [Crossref] [PubMed]
- Fang X, Palanivel R, Cresser J, et al. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab 2010;299:E721-9. [Crossref] [PubMed]
- Palanivel R, Eguchi M, Shuralyova I, et al. Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism 2006;55:1067-75. [Crossref] [PubMed]
- Onay-Besikci A, Altarejos JY, Lopaschuk GD. gAd-globular head domain of adiponectin increases fatty acid oxidation in newborn rabbit hearts. J Biol Chem 2004;279:44320-6. [Crossref] [PubMed]
- Piñeiro R, Iglesias MJ, Gallego R, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 2005;579:5163-9. [Crossref] [PubMed]
- Belke DD, Larsen TS, Gibbs EM, et al. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 2000;279:E1104-13. [Crossref] [PubMed]
- Noh HL, Yamashita H, Goldberg IJ. Cardiac metabolism and mechanics are altered by genetic loss of lipoprotein triglyceride lipolysis. Cardiovasc Drugs Ther 2006;20:441-4. [Crossref] [PubMed]
- Li X, Zhang D, Vatner DF, et al. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci U S A 2020;117:32584-93. [Crossref] [PubMed]
- Kobayashi J, Saito K, Fukamachi I, et al. Pre-heparin plasma lipoprotein lipase mass: correlation with intra-abdominal visceral fat accumulation. Horm Metab Res 2001;33:412-6. [Crossref] [PubMed]
- Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Prospective Cardiovascular Münster study. Am J Cardiol 1992;70:733-7. [Crossref] [PubMed]
- Shang R, Rodrigues B. Lipoprotein Lipase and Its Delivery of Fatty Acids to the Heart. Biomolecules 2021;11:1016. [Crossref] [PubMed]
- Kim MS, Wang Y, Rodrigues B. Lipoprotein lipase mediated fatty acid delivery and its impact in diabetic cardiomyopathy. Biochim Biophys Acta 2012;1821:800-8. [Crossref] [PubMed]
- An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 2006;291:H1489-506. [Crossref] [PubMed]
- Shimada M, Ishibashi S, Inaba T, et al. Overexpression of lipoprotein lipase reduced atherosclerotic lesions in low density lipoprotein receptor deficient mice. Circulation 1995;92:359-64.
- Rip J, Nierman MC, Wareham NJ, et al. Serum lipoprotein lipase concentration and risk for future coronary artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol 2006;26:637-42. [Crossref] [PubMed]
- Ferguson MA, Alderson NL, Trost SG, et al. Effects of four different single exercise sessions on lipids, lipoproteins, and lipoprotein lipase. J Appl Physiol (1985) 1998;85:1169-74. [PubMed]
- Gill JM, Herd SL, Vora V, et al. Effects of a brisk walk on lipoprotein lipase activity and plasma triglyceride concentrations in the fasted and postprandial states. Eur J Appl Physiol 2003;89:184-90. [Crossref] [PubMed]
- Seip RL, Mair K, Cole TG, et al. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am J Physiol 1997;272:E255-61. [PubMed]
- Miyashita M, Tokuyama K. Moderate exercise reduces serum triacylglycerol concentrations but does not affect pre-heparin lipoprotein lipase concentrations after a moderate-fat meal in young men. Br J Nutr 2008;99:1076-82. [Crossref] [PubMed]
- TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22-31. [Crossref] [PubMed]
- CARDIoGRAMplusC4D Consortium; Deloukas P, Kanoni S, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013;45:25-33.
- Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol 2015;65:2267-75. [Crossref] [PubMed]
- Chait A, Eckel RH. The Chylomicronemia Syndrome Is Most Often Multifactorial: A Narrative Review of Causes and Treatment. Ann Intern Med 2019;170:626-34. [Crossref] [PubMed]
- Olsson AG, Lang PD. Dose-response study of bezafibrate on serum lipoprotein concentrations in hyperlipoproteinanemia. Atherosclerosis 1978;31:421-8. [Crossref] [PubMed]
- Olsson AG, Lang PD. One-year study of the effect of bezafibrate on serum lipoprotein concentrations in hyperlipoproteinaemia. Atherosclerosis 1978;31:429-33. [Crossref] [PubMed]
- Saiki A, Miyashita Y, Shirai K. The role of pitavastatin-enhanced lipoprotein lipase expression in 3T3-L1 preadipocytes. J Atheroscler Thromb 2006;13:122. [Crossref] [PubMed]
- Ohira M, Endo K, Saiki A, et al. Atorvastatin and pitavastatin enhance lipoprotein lipase production in L6 skeletal muscle cells through activation of adenosine monophosphate-activated protein kinase. Metabolism 2012;61:1452-60. [Crossref] [PubMed]
- Do R, Stitziel NO, Won HH, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015;518:102-6. [Crossref] [PubMed]
- Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 2008;247:401-7. [Crossref] [PubMed]
- Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg 2014;149:275-87. [Crossref] [PubMed]
- Näsström B, Olivecrona G, Olivecrona T, et al. Lipoprotein lipase during heparin infusion: lower activity in hemodialysis patients. Scand J Clin Lab Invest 2003;63:45-53. [Crossref] [PubMed]
- Näsström B, Olivecrona G, Olivecrona T, et al. Lipoprotein lipase during continuous heparin infusion: tissue stores become partially depleted. J Lab Clin Med 2001;138:206-13. [Crossref] [PubMed]
Cite this article as: Kimura T, Tsunekawa K, Nagasawa T, Aoki T, Miyashita K, Yoshida A, Nakajima K, Murakami M. Circulating levels of lipoprotein lipase and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1: new markers for cardiovascular diseases among noncommunicable diseases: a brief narrative review. J Lab Precis Med 2023;8:18.


