
Can cellular and humoral immunity predict response to BNT162b2 bivalent booster?
Predicting response to coronavirus disease 2019 (COVID-19) vaccination is an important basis for future interventions to combat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the foreseeable endemic phase, since ensuring sufficient humoral and cellular immunity is critical to minimizing the impact of the virus on the more susceptible segments of the population (1). To this end, we conducted a retrospective analysis of an ongoing serosurveillance protocol (2), to determine whether cellular or natural immunity can be used as a reliable marker of response to administration of the new bivalent COVID-19 vaccines.
We retrospectively analyzed 51 healthcare employees of the Hospital Pederzoli (Peschiera del Garda, Verona, Italy) previously vaccinated and boosted with the Pfizer/BioNTech mRNA monovalent BNT162b2 vaccine (Comirnaty, Pfizer Inc., NY, USA), before and 15 days after receiving a single BNT162b2 bivalent booster (Comirnaty, Pfizer Inc.) ≥1 year after the last monovalent dose of vaccine. Basal and post-bivalent booster immunity was assayed using the specific SARS-CoV-2 Cobas interferon gamma release assay (IGRA), and by measuring total anti-SARS-CoV-2 antibodies with Roche Elecsys (Roche Diagnostics, Basel, Switzerland) anti-SARS-CoV-2 electrochemiluminescence immunoassay (ECLIA). Positive response after BNT162b2 bivalent vaccine administration was defined as positive variation from the pre-vaccination value. Test results were analyzed with Analyse-it (Analyse-it Software Ltd., Leeds, UK). All subjects provided a written informed consent for participating to the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Verona and Rovigo Provinces (59COVIDCESC; November 8, 2021).
Both IGRA and total anti-SARS-CoV-2 antibodies levels increased after bivalent vaccine booster from 1.00±1.62 to 1.07±0.90 ng/mL (P=0.385), and from 12,193±7,646 to 21,530±5,060 kU/L (P<0.001), respectively. A positive IGRA response (i.e., >0.013 ng/mL) was detected in 44/51 (86.3%) and 50/51 (98.0%) subjects before and after vaccination, respectively (chi-square, 3.391; P=0.033). Instead, the serum of all subjects was reactive for total anti-SARS-CoV-2 antibodies both before and after vaccination (i.e., >0.8 kU/L: 51/51; 100%). In receiver operating characteristic (ROC) curve analysis, SARS-CoV-2 IGRA was a poor predictor of response to bivalent BNT162b2 vaccine [area under the curve (AUC), 0.63; 95% CI: 0.52–0.74; P=0.010], whereas better performance was observed for total anti-SARS-CoV-2 antibodies (AUC, 0.83; 95% CI: 0.75–0.91; P<0.001) (Figure 1). A threshold 11,723 kU/L for total anti-SARS-CoV-2 antibodies was associated with 0.96 sensitivity and a specificity of 0.59 for predicting response to bivalent BNT162b2 vaccine.
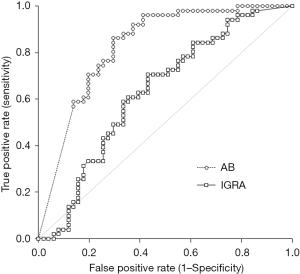
The results of this retrospective observational study suggest that SARS-CoV-2 IGRA may be a poor predictor of response to bivalent BNT162b2 vaccine in healthy individuals, likely because basal T cell immunity levels are already elevated at baseline due to repeated vaccination and/or natural infection. In contrast, monitoring of total anti-SARS-CoV-2 antibodies remains a better option for this purpose.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-36/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-36/coif). GL serves as the Editor-in-Chief of Journal of Laboratory and Precision Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All subjects provided a written informed consent for participating to the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Verona and Rovigo Provinces (No. 59COVIDCESC; November 8, 2021).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Callaway E. COVID's future: mini-waves rather than seasonal surges. Nature 2023;617:229-30. [Crossref] [PubMed]
- Salvagno GL, Pighi L, Henry BM, et al. Assessment of humoral and cellular immunity after bivalent BNT162b2 vaccination and potential association with reactogenicity. Clin Chem Lab Med 2023;61:1343-8. [Crossref] [PubMed]
Cite this article as: Pighi L, Henry BM, De Nitto S, Salvagno GL, Lippi G. Can cellular and humoral immunity predict response to BNT162b2 bivalent booster? J Lab Precis Med 2023;8:24.


