
Ruling out acute myocardial infarction based on a single high-sensitivity troponin measurement in the emergency department: a clinical practice review
Introduction
Background
Emergency departments (EDs) around the world are increasingly overcrowded, with excess numbers of patients and long wait times, both associated with significant patient harm (1-3). Treatment delays increase morbidity and mortality for both low- and high-acuity patients (4,5). Additionally, rates of preventable error increase (6), additional patients who require urgent assessment and treatment leave without being seen by a doctor (7,8), and ambulances are diverted (9).
Access block has been defined as: ‘the situation where patients are unable to gain access to appropriate hospital beds within a reasonable amount of time, no greater than 8 hours’ (10). It is a significant contributor to overcrowding, and leads to longer hospital stays and higher costs (11-13). ED overcrowding is also associated with increased violence and aggression towards staff, staff turnover, staff distraction leading to error (14,15), and contributes to high levels of burnout in emergency physicians (16). Patient experiences are also poor, with patients being cared for on trolleys in corridors or in the ED waiting room (17).
Public awareness campaigns around the world encourage patients with symptoms potentially associated with myocardial infarction to promptly seek medical attention. As a result, acute chest pain is one of the most common presentations to EDs, accounting for between 5–10% of ED attendances (18-20), and with hospital admission rates of 40–70% (21,22), although the numbers of patients with acute myocardial infarction (AMI) are relatively low (23-25). Mortality rates for all acute chest pain presentations are approximately 1–2%, however there is significant variation depending on the underlying cause and patient demographics (26,27).
Missed heart attack carries risk. There are many reasons for chest pain, some life-threatening, however only a minority will be eventually diagnosed with an acute coronary syndrome (ACS). This means that the assessment and safe decision making for the management of these patients is challenging and time-consuming. Patients presenting to ED with chest pain are assessed for AMI, and if this can confidently be ruled out they may be discharged from ED. Patients who are considered at high risk for underlying coronary artery disease (CAD) as a cause for their symptoms may require further investigation (19).
Although the development of protocolized chest pain pathways using serial troponin assays have decreased the time that patients with chest pain spend in ED, the absolute number of these presentations (28) means that the burden on the health system remains significant, and even small improvements in ED or hospital length of stay can reduce overcrowding and improve health care for all those presenting to EDs (29,30).
The diagnosis of acute myocardial infarction cannot be made on either cTn alone, or with a single result. Dynamic cTn concentration change is required in the clinical context of myocardial ischaemia, identified by symptoms suggestive of AMI, electrocardiogram (ECG) changes or imaging including coronary angiography (19,31). AMI may be further defined as Type 1, related to coronary artery plaque rupture and superimposed thrombosis or Type 2, characterised by an imbalance between myocardial oxygen supply and demand, such as in sepsis or pulmonary embolism (31), however this differentiation is not always clear during a short ED visit, and often requires further testing as an inpatient. Acute myocardial injury, with rising and/or falling concentrations of cTn, can also be differentiated from chronic myocardial injury with static concentrations about the 99th percentile (31). In this review with use the term ‘acute myocardial infarction’ or AMI to describe both type 1 and type 2 MI.
Rational and knowledge gap
Cardiac troponin (cTn) (I or T) is endorsed by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), the National Academy of Clinical Biochemistry (NACB) and expert societies worldwide as the preferred biomarker for the assessment of possible ACS (31-35), with clinical guidelines recommending a turn-around time (TAT) of less than 60 minutes for high sensitivity troponin assays (hs-cTn) (32,34,35). Although ED overcrowding is multifactorial, a delayed time between a sample being taken and the results being available for the clinician to act on the result can be considerably longer than the lab TAT (36). This has been cited as a contributing factor to ED overcrowding (37,38).
ED clinicians and clinical biochemists (39) may view cardiac troponin differently. Clinical biochemists focus on the analytical performance of a test, whereas emergency physicians focus on the clinical predictive performance use of the test, particularly in relation to accurate ‘rule-out’ of AMI. Cardiologists (40,41) focus on the accurate identification of patients with AMI secondary to coronary artery disease (as opposed to other causes of an increase in troponin) who may benefit from procedural or therapeutic interventions.
When assessing patients with cardiac chest pain, the primary goal of the emergency physician is to rapidly identify those patients at low risk of AMI to expediate discharge, while ensuring that those at high risk receive prompt treatment and referral to cardiology or other inpatient teams. Single test rule out strategies involve decision making at very low troponin concentration thresholds, and therefore emergency physicians require assays with accuracy at low cTn concentrations.
Objective
The objective of this review is to summarise the development of decision-making pathways for the assessment of chest pain, and describe the current changes taking place in pathway use involving single test rule out of AMI in the ED. The purpose of this clinical practice review is to provide clinical biochemists with an understanding of how emergency clinicians use and approach cardiac troponin results within the ED.
How high-sensitivity troponin assays are used within the emergency department
Characteristics of high-sensitivity troponin assays
cTn assays are becoming increasingly sensitive, with the ability to precisely measure cTn at very low concentrations, allowing for the earlier rule-out of myocardial injury. Hs-cTn assays are characterised by the detection of cTn in 50% of healthy individuals below the 99th percentile, with a coefficient of variation (CV) of 10% or less at the 99th percentile (33,42). Compared to previous lower sensitivity (contemporary) assays, hs-cTn assays allow for earlier recognition of a rise and/or fall in concentration, and therefore the rapid rule-out of ACS through accelerated diagnostic chest pain algorithms (21,29,35,43). It is important to note that there is strong evidence that patients need to be tested at least 3 hours after onset of symptoms (19,34,44). High-sensitivity troponin assays also allow for the use of gender-specific upper reference limits (35,43,45).
Use of high-sensitivity troponin assays in clinical decision making
There is a rule-out threshold lower than the 99th centile that is specific to each hs-cTn assay. Application of these cTn thresholds in decision-making pathways used to rule out myocardial infarction will miss fewer cardiac events than those relying on the 99th centile (46). When deriving the hs-cTn threshold concentration for use in a chest pain decision pathway, both the (statistical) sensitivity and negative predictive value (NPV) of the assay at a threshold must be considered.
The prevalence of ACS in ED studies varies greatly from 1% to over 20% (23-25). A high NPV is easily achieved (for a pathway designed to rule-out ACS) in a population with a very low prevalence of disease. Data presented by Mahler et al. (23) using the History, ECG, Age, Risk factors, and initial Troponin (HEART) score to rule out ACS demonstrates that with a low prevalence of disease (1.1%), there is a very high NPV (99.4%), but an unsafe sensitivity (58.3%). Therefore, the assay characteristics must be considered in the context of the disease prevalence of the population within which the test is being used.
The sensitivity of a threshold is the most important consideration. A survey of over 1,000 emergency medicine physicians from Australasia, United States of America and Canada found that the most acceptable rate of error for a missed diagnosis of AMI was between ≤1 in 100 (1%) and ≤1 in 1,000 (0.1%) in patients discharged from ED. This suggests that a sensitivity of 99% or higher is required for a test comprising a low cTn threshold, a risk scoring system, and an electrocardiogram, which allows for the early discharge of chest pain patients from ED (47).
Development of accelerated diagnostic pathways (ADPs)
The traditional management of patients presenting to EDs with potential cardiac chest pain was to admit the majority of patients to hospital, allowing for serial biomarker testing. A delay of up to six to nine hours between samples was previously needed as contemporary sensitive troponin assays could not safely rule out AMI at presentation (48). With an assumed prevalence rate of ACS of approximately 20%, Figure 1 demonstrates that up to 70% of patients would be unnecessarily admitted to hospital for prolonged observation and other investigations (such as exercise stress test or angiography) (see Figure 1, pre-ADPs).
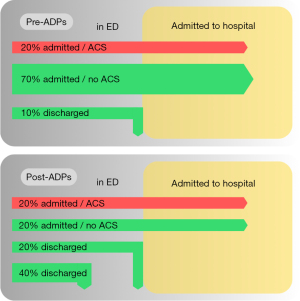
With the advent of hs-cTn assays, and increasing knowledge around the risk stratification of patients with chest pain, ADPs have been developed with the aim of identifying patients at low and intermediate risk of ACS, who can have ACS ruled-out and therefore discharged from ED at earlier time points (see Figure 1, post-ADPs).
ADPs are designed to enable clinicians to quickly and accurately identify patients who are at low risk of acute myocardial infarction, and who therefore can be safely discharged, whilst ensuring that high-risk patients receive prompt and appropriate care.
Five approaches have been taken to the development of ADPs.
- The use of clinician gestalt plus a risk stratification algorithm based on troponin alone. This was the approach of the 0/1 h and 0/2 h algorithms in the European Society of Cardiology guidelines (34).
- A score made-up by an expert(s) that incorporates troponin. This was the approach taken for the development of the HEART score (23,49), incorporated within a pathway including serial troponin (50).
- Modification of a pre-existing risk score, Thrombolysis In Myocardial Infarction (TIMI) (51), related to mortality outcomes following MI in conjunction with ECG and troponins, the ADAPT ADP (52).
- Development of a risk score that predicts major adverse cardiac events (MACE) with signs, symptoms, demographics, and patient history on presentation to the ED, Emergency Department Assessment of Chest Pain Score (EDACS) used in conjunction with ECG and troponin (53).
- Development of a statistical/machine learning algorithm which predicts the likelihood of MI based on troponin and other variables gathered on presentation to ED, Troponin-only Manchester Acute Coronary Syndromes (T-MACS) (54), MI3 (55), CoDE-ACS (25).
Until recently, a serial (double) test rule out within an ADP was standard of care, however the precision of hs-cTn assays have improved, and when an hs-cTn assay is used in conjunction with an appropriate ADP, it is now possible to rule-out AMI in a proportion of low-risk patients with hs-cTn concentrations close to or below the limit of detection (LoD).
Single test rule-out of acute myocardial infarction
The aim of a single test rule-out pathway is to identify patients at such low risk of having myocardial infarction that they don’t require a second test, allowing early discharge from the ED. A number of studies have demonstrated that very low hs-cTn concentrations at presentation can be used to risk stratify patients (56-58), with concentrations well below the 99th percentile upper reference limit (URL) able to identify those at very-low risk of an AMI. A prospective study of 4,870 patients with possible ACS (of whom 16% had AMI) demonstrated that an hs-cTn concentration ≤5 ng/L at presentation had an NPV of 99.6% (95% CI: 99.3–99.8%) for AMI, or MI or death within 30 days (59). This threshold enabled the identification and discharge of two-thirds of the patients without an AMI after a single test. Caution is noted for those patients who present within 2 hours of onset of pain, where repeat testing is recommended as the NPV of a single test is lower, in this study in these patients 97.6% (95% CI: 95.8–99.2%).
The possibility of using a single blood draw with a low-concentration of cTn to rule-out AMI was raised with the advent of hs-cTn assays (56). Two large international meta-analyses of the performance of single test rule-out thresholds, one for the Roche hs-cTnT assay (44) and one for the Abbott hs-cTnI assay (60), provided the evidence on safety and performance needed for adoption of these thresholds into clinical practice. Across 11 cohorts and 9,241 participants a low-risk hs-cTnT test of <5 ng/L (i.e., at the LoD of the assay) and no-new ischaemia on ECG classified 30.6% as low-risk with an NPV of 99.3% (95% CI: 97.3–99.8%) and sensitivity of 98.7% (95% CI: 96.6–99.5%) for AMI. Across 19 cohorts and 22,457 participants a low-risk hs-cTnI test of <5 ng/L (3 ng/L above the LoD) classified 49.0% low-risk with an NPV of 99.5% (95% CI: 99.3–99.6%) for 30-day AMI. Both of these studies observed that approximately 50% of the missed cases occurred in participants in whom the blood draw was within 3 hours of symptom onset.
For most other assays, a single-test rule-out threshold has been established simply by finding in a cohort the threshold that if applied clinically would have resulted in an NPV ≥99.5% or sensitivity ≥99.0%. The safety of these threshold are validated in other cohorts. Whilst sometimes called optimal thresholds, they are almost always based on small numbers of AMIs (sometimes <100) which means that they are dependent on the exact concentrations of just one or two patients (61). By analogy, this is similar to a process to determine a 99th centile with just 100 subjects. Despite the inherent imprecision in these threshold estimates, their safety record is strong and in most jurisdictions, where prevalence is not high, they enable rule-out of AMI in a substantial proportion of patients.
A single troponin test for rule-out of ACS should only be used within ADPs. There are several single-test rule-out pathways in use globally. Commonly used pathways include the:
- EDACS pathway (62) (see Figure S1).
EDACS pathways were developed in Australasia and are commonly used in this region (63). They combine a structured risk assessment (EDACS) with ECG findings, time from symptom onset, and the presence of unstable features such as crescendo angina or abnormal vital signs to determine which patients, and which troponin threshold, can be used for single-test and serial-test rule-out of AMI (64). - European Society of Cardiology (ESC) pathway (see Figure S2).
The ESC-based pathways recommend the use of clinical judgement and ECG interpretation in combination with an algorithmic troponin threshold approach. The ESC guidance provides assay-specific threshold recommendations for single-test rule-out. The ESC pathway was developed in Switzerland and has a good European following. Both this and the EDACS pathway have some additional usage beyond their own regions. - HEART score-based pathways (50) (see Figure S3).
Pathways based on the HEART score probably have the widest usage internationally, particularly in the USA. The HEART score was originally created in The Netherlands (49). Variants that use single-test for troponin test rule-out are often referred to as HEAR pathways (representing history, ECG, age and risk factors) which are then combined with troponin thresholds. - High-Sensitivity Troponin in the Evaluation of patients with suspected Acute Coronary Syndrome (High-STEACS) (59) (see Figure S4).
The High-STEACS pathway was developed in Edinburgh and is widely used in the local region. The High-STEACS pathway developers have troponin-specific thresholds for single-test rule-out and the pathway does not incorporate clinical findings and examination findings as long as the patient is stable. - T-MACS pathway (54).
The T-MACS pathway combines a mathematical algorithm of clinical findings with troponin results within a calculator to predict patient likelihood of AMI and provides guidance about probability (54).
All of these pathways have been validated in real-life patient care, are considered safe to use, and have been shown to be effective in facilitating earlier discharge of patients from the ED (34,50,54,59,62,63). Shah et al. (65) showed this to be the case in over 31,000 patients.
Some countries or regions, e.g., New Zealand and several Australian states, have developed a cross-system consistent approach to patient assessment. In New Zealand, for example, every hospital has a single-test rule out approach within its AMI assessment pathway. The most common approach in New Zealand is based upon the EDACS pathway however a small number of centres also use an ESC-based approach.
The place of point-of-care (POC)
POC troponin testing, performed by clinical staff at or near the site of the patient, are able to significantly reduce TAT by reducing specimen transport and handling time. POC testing allows for results to the treating clinician within 20 minutes or less (66-71), as well as testing in a range of healthcare environments, such as ambulances or rural clinics distant from laboratories. Rapid test results can allow rapid decision making to occur in conjunction with ADPs, which can reduce ED length of stay and therefore promote better outcomes for low-risk patients who only require a single troponin for rule-out of AMI.
The limiting factor in the use of POC troponin testing in clinical practice until recently is that POC and central laboratory assays do not show equivalent analytical precision. New POC hs-Tn assays are now beginning to demonstrate comparable precision to laboratory-based assays (72,73). Using a validated very low concentration threshold in patents presenting at least 2–3 hours after the onset of symptoms these may allow the rule-out of AMI (34) after only one blood sample (66,67,74). Such high sensitivity POC cTn assays have only just begun to be used in EDs for clinical decision-making. They have the potential to significantly reduce TAT and ED length of stay and overcrowding.
Single test “rule-in”
A single test does not allow for a detection of a rise and/or fall in cardiac biomarkers; a requirement for the diagnosis of AMI. One hs-cTn result above the 99th percentile does not differentiate between acute and chronic myocardial injury, and does not allow for the determination of a Type 1 versus Type 2 AMI. Further testing such as angiography demonstrating critical coronary artery stenosis or imaging providing evidence of new regional wall motion abnormalities are often required to make a conclusive diagnosis of the cause for a cTn rise (and therefore determination of a Type 1 versus Type 2 AMI). Such testing is not usually available in EDs, and so a single test rule-in strategy is rarely feasible outside the context of an ST elevation AMI. Some risk-stratification algorithms, such as those recommended in the ESC guidelines, use an elevated (above the URL) troponin threshold and the term “rule-in” to risk stratify patients into a high-risk group where they may receive more immediate attention from cardiologists.
Strengths and limitations
The principle strength of this clinical practice review is that it has been written predominantly by emergency physicians, and so provides an ED perspective on the use of single test rule out for AMI. It focusses on the experience of assessing patients with chest pain in ED, rather than from a cardiology (40,41) or laboratory (39) viewpoint; consequently a limitation is that the views of these groups are not emphasised.
Conclusions
High sensitivity troponin assays have improved precision at measuring low concentrations of circulating troponin compared to contemporary assays. This allows the identification of patients at very low probability of having AMI that don’t require repeat testing. When used within a structured decision making pathway, this facilitates safe early discharge from the ED.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Xander van Wijk, Amy Saenger, Steven Meex and Allan Jaffe) for the series “Cardiac Troponin” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-20/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-20/coif). The series “Cardiac Troponin” was commissioned by the editorial office without any funding or sponsorship. MT reports grants and speaker fees and research and education support from Abbott, Alere, Beckman Radiometer, Roche and Siemens outside the submitted work. JWP reports no conflicts directly related to the content of the manuscript. Other biostatistics and advisory board work for cardiac biomarker companies. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morley C, Unwin M, Peterson GM, et al. Emergency department crowding: A systematic review of causes, consequences and solutions. PLoS One 2018;13:e0203316. [Crossref] [PubMed]
- Verelst S, Wouters P, Gillet JB, et al. Emergency Department Crowding in Relation to In-hospital Adverse Medical Events: A Large Prospective Observational Cohort Study. J Emerg Med 2015;49:949-61. [Crossref] [PubMed]
- Jones PG, van der Werf B. Emergency department crowding and mortality for patients presenting to emergency departments in New Zealand. Emerg Med Australas 2021;33:655-64. [Crossref] [PubMed]
- Chalfin DB, Trzeciak S, Likourezos A, et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med 2007;35:1477-83. [Crossref] [PubMed]
- Berg LM, Ehrenberg A, Florin J, et al. Associations Between Crowding and Ten-Day Mortality Among Patients Allocated Lower Triage Acuity Levels Without Need of Acute Hospital Care on Departure From the Emergency Department. Ann Emerg Med 2019;74:345-56. [Crossref] [PubMed]
- Epstein SK, Huckins DS, Liu SW, et al. Emergency department crowding and risk of preventable medical errors. Intern Emerg Med 2012;7:173-80. [Crossref] [PubMed]
- Wiler JL, Bolandifar E, Griffey RT, et al. An emergency department patient flow model based on queueing theory principles. Acad Emerg Med 2013;20:939-46. [Crossref] [PubMed]
- Bair AE, Song WT, Chen YC, et al. The impact of inpatient boarding on ED efficiency: a discrete-event simulation study. J Med Syst 2010;34:919-29. [Crossref] [PubMed]
- Shen YC, Hsia RY. Association between ambulance diversion and survival among patients with acute myocardial infarction. JAMA 2011;305:2440-7. [Crossref] [PubMed]
- Australasian College for Emergency Medicine. Position Statement on Access Block [Internet]. 2022 Oct. Available online: https://acem.org.au/getmedia/c0bf8984-56f3-4b78-8849-442feaca8ca6/S127_v01_Statement_Access_Block_Mar_14.aspx
- McCarthy ML, Zeger SL, Ding R, et al. Crowding delays treatment and lengthens emergency department length of stay, even among high-acuity patients. Ann Emerg Med 2009;54:492-503.e4. [Crossref] [PubMed]
- White BA, Biddinger PD, Chang Y, et al. Boarding inpatients in the emergency department increases discharged patient length of stay. J Emerg Med 2013;44:230-5. [Crossref] [PubMed]
- Kobayashi KJ, Knuesel SJ, White BA, et al. Impact on Length of Stay of a Hospital Medicine Emergency Department Boarder Service. J Hosp Med 2020;15:147-53. [Crossref] [PubMed]
- Medley DB, Morris JE, Stone CK, et al. An association between occupancy rates in the emergency department and rates of violence toward staff. J Emerg Med 2012;43:736-44. [Crossref] [PubMed]
- Levin S, Sauer L, Kelen G, et al. Situation awareness in emergency medicine. IIE Transactions on Healthcare Systems Engineering 2012;2:172-80. [Crossref]
- Shanafelt TD, West CP, Sinsky C, et al. Changes in Burnout and Satisfaction With Work-Life Integration in Physicians and the General US Working Population Between 2011 and 2017. Mayo Clin Proc 2019;94:1681-94. [Crossref] [PubMed]
- Reznek MA, Larkin CM, Scheulen JJ, et al. Operational factors associated with emergency department patient satisfaction: Analysis of the Academy of Administrators of Emergency Medicine/Association of Academic Chairs of Emergency Medicine national survey. Acad Emerg Med 2021;28:753-60. [Crossref] [PubMed]
- Dawson LP, Smith K, Cullen L, et al. Care Models for Acute Chest Pain That Improve Outcomes and Efficiency: JACC State-of-the-Art Review. J Am Coll Cardiol 2022;79:2333-48. [Crossref] [PubMed]
- Writing Committee Members. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;78:e187-e285. [Crossref] [PubMed]
- Cullen L, Greenslade J, Merollini K, et al. Cost and outcomes of assessing patients with chest pain in an Australian emergency department. Med J Aust 2015;202:427-32. [Crossref] [PubMed]
- Chew DP, Lambrakis K, Blyth A, et al. A Randomized Trial of a 1-Hour Troponin T Protocol in Suspected Acute Coronary Syndromes: The Rapid Assessment of Possible Acute Coronary Syndrome in the Emergency Department With High-Sensitivity Troponin T Study (RAPID-TnT). Circulation 2019;140:1543-56. [Crossref] [PubMed]
- Stoyanov KM, Hund H, Biener M, et al. RAPID-CPU: a prospective study on implementation of the ESC 0/1-hour algorithm and safety of discharge after rule-out of myocardial infarction. Eur Heart J Acute Cardiovasc Care 2020;9:39-51. [Crossref] [PubMed]
- Mahler SA, Hiestand BC, Goff DC Jr, et al. Can the HEART score safely reduce stress testing and cardiac imaging in patients at low risk for major adverse cardiac events? Crit Pathw Cardiol 2011;10:128-33. [Crossref] [PubMed]
- Pickering JW, Young JM, George PM, et al. Early kinetic profiles of troponin I and T measured by high-sensitivity assays in patients with myocardial infarction. Clin Chim Acta 2020;505:15-25. [Crossref] [PubMed]
- Doudesis D, Lee KK, Boeddinghaus J, et al. Machine learning to optimise cardiac troponin for the diagnosis of myocardial infarction. Nat Med 2023; In Press. [Crossref]
- Pedersen CK, Stengaard C, Friesgaard K, et al. Chest pain in the ambulance; prevalence, causes and outcome - a retrospective cohort study. Scand J Trauma Resusc Emerg Med 2019;27:84. [Crossref] [PubMed]
- Andrew E, Cox S, Smith K. Linking Ambulance Records with Hospital and Death Index Data to Evaluate Patient Outcomes. Int J Gen Med 2022;15:567-72. [Crossref] [PubMed]
- Pines JM, Pollack CV Jr, Diercks DB, et al. The association between emergency department crowding and adverse cardiovascular outcomes in patients with chest pain. Acad Emerg Med 2009;16:617-25. [Crossref] [PubMed]
- Than M, Aldous S, Lord SJ, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med 2014;174:51-8. [Crossref] [PubMed]
- Vigen R, Diercks DB, Hashim IA, et al. Association of a Novel Protocol for Rapid Exclusion of Myocardial Infarction With Resource Use in a US Safety Net Hospital. JAMA Netw Open 2020;3:e203359. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018;138:e618-e651. Correction in Circulation. 2018;138:e652.
- Christenson RH, Azzazy HM. Cardiac point of care testing: a focused review of current National Academy of Clinical Biochemistry guidelines and measurement platforms. Clin Biochem 2009;42:150-7. [Crossref] [PubMed]
- Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61. [Crossref] [PubMed]
- Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. [Crossref] [PubMed]
- Tan JWC, Lam CSP, Kasim SS, et al. Asia-Pacific consensus statement on the optimal use of high-sensitivity troponin assays in acute coronary syndromes diagnosis: focus on hs-TnI. Heart Asia 2017;9:81-7. [Crossref] [PubMed]
- Lundberg GD. Adding outcome as the 10th step in the brain-to-brain laboratory test loop. Am J Clin Pathol 2014;141:767-9. [Crossref] [PubMed]
- Affleck A, Parks P, Drummond A, et al. Emergency department overcrowding and access block. CJEM 2013;15:359-84. [Crossref] [PubMed]
- Bukhari H, Albazli K, Almaslmani S, et al. Analysis of Waiting Time in Emergency Department of Al-Noor Specialist Hospital, Makkah, Saudi Arabia. Open Journal of Emergency Medicine 2014;2:67-73. [Crossref]
- Wu AHB, Christenson RH, Greene DN, et al. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645-55. [Crossref] [PubMed]
- Lopez-Ayala P, Boeddinghaus J, Koechlin L, et al. Early Rule-Out Strategies in the Emergency Department Utilizing High-Sensitivity Cardiac Troponin Assays. Clin Chem 2021;67:114-23. [Crossref] [PubMed]
- Kontos MC, Turlington JS. High-Sensitivity Troponins in Cardiovascular Disease. Curr Cardiol Rep 2020;22:30. [Crossref] [PubMed]
- Apple FS. A new season for cardiac troponin assays: it's time to keep a scorecard. Clin Chem 2009;55:1303-6. [Crossref] [PubMed]
- Kozinski M, Krintus M, Kubica J, et al. High-sensitivity cardiac troponin assays: From improved analytical performance to enhanced risk stratification. Crit Rev Clin Lab Sci 2017;54:143-72. [Crossref] [PubMed]
- Pickering JW, Than MP, Cullen L, et al. Rapid Rule-out of Acute Myocardial Infarction With a Single High-Sensitivity Cardiac Troponin T Measurement Below the Limit of Detection: A Collaborative Meta-analysis. Ann Intern Med 2017;166:715-24. [Crossref] [PubMed]
- Cullen LA, Mills NL. Point: The Use of Sex-Specific Cutpoints for High-Sensitivity Cardiac Troponin Assays. Clin Chem 2017;63:261-3. [Crossref] [PubMed]
- Chapman AR, Fujisawa T, Lee KK, et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart 2019;105:616-22. [Crossref] [PubMed]
- Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department?: a clinical survey. Int J Cardiol 2013;166:752-4. [Crossref] [PubMed]
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525-38. [Crossref] [PubMed]
- Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J 2008;16:191-6. [Crossref] [PubMed]
- Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes 2015;8:195-203. [Crossref] [PubMed]
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000;284:835-42. [Crossref] [PubMed]
- Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012;59:2091-8. [Crossref] [PubMed]
- Than MP, Pickering JW, Aldous SJ, et al. Effectiveness of EDACS Versus ADAPT Accelerated Diagnostic Pathways for Chest Pain: A Pragmatic Randomized Controlled Trial Embedded Within Practice. Ann Emerg Med 2016;68:93-102.e1. [Crossref] [PubMed]
- Body R, Carlton E, Sperrin M, et al. Troponin-only Manchester Acute Coronary Syndromes (T-MACS) decision aid: single biomarker re-derivation and external validation in three cohorts. Emerg Med J 2017;34:349-56. [Crossref] [PubMed]
- Than MP, Pickering JW, Sandoval Y, et al. Machine Learning to Predict the Likelihood of Acute Myocardial Infarction. Circulation 2019;140:899-909. [Crossref] [PubMed]
- Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332-9. [Crossref] [PubMed]
- Bandstein N, Ljung R, Johansson M, et al. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol 2014;63:2569-78. [Crossref] [PubMed]
- Sandoval Y, Lewis BR, Mehta RA, et al. Rapid Exclusion of Acute Myocardial Injury and Infarction With a Single High-Sensitivity Cardiac Troponin T in the Emergency Department: A Multicenter United States Evaluation. Circulation 2022;145:1708-19. [Crossref] [PubMed]
- Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8. [Crossref] [PubMed]
- Chapman AR, Lee KK, McAllister DA, et al. Association of High-Sensitivity Cardiac Troponin I Concentration With Cardiac Outcomes in Patients With Suspected Acute Coronary Syndrome. JAMA 2017;318:1913-24. [Crossref] [PubMed]
- Pickering JW. The Need to Improve Derivation and Description of Algorithms to Rule-Out Patients With Possible Myocardial Infarction. Circulation 2019;139:1351-3. [Crossref] [PubMed]
- Than M, Flaws D, Sanders S, et al. Development and validation of the Emergency Department Assessment of Chest pain Score and 2 h accelerated diagnostic protocol. Emerg Med Australas 2014;26:34-44. [Crossref] [PubMed]
- Than MP, Pickering JW, Dryden JM, et al. ICare-ACS (Improving Care Processes for Patients With Suspected Acute Coronary Syndrome): A Study of Cross-System Implementation of a National Clinical Pathway. Circulation 2018;137:354-63. [Crossref] [PubMed]
- Than MP, Pickering JW, Adamson P, et al. Reducing Patient Risk and Enhancing Care Through the Development and Implementation of a New Chest Pain Pathway, Expedited by and for the COVID-19 Era. EJIFCC 2021;32:27-40. [PubMed]
- Shah ASV, Anand A, Strachan FE, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919-28. [Crossref] [PubMed]
- Boeddinghaus J, Nestelberger T, Koechlin L, et al. Early Diagnosis of Myocardial Infarction With Point-of-Care High-Sensitivity Cardiac Troponin I. J Am Coll Cardiol 2020;75:1111-24. [Crossref] [PubMed]
- Sörensen NA, Neumann JT, Ojeda F, et al. Diagnostic Evaluation of a High-Sensitivity Troponin I Point-of-Care Assay. Clin Chem 2019;65:1592-601. [Crossref] [PubMed]
- Apple FS, Schulz K, Schmidt CW, et al. Determination of sex-specific 99th percentile upper reference limits for a point of care high sensitivity cardiac troponin I assay. Clin Chem Lab Med 2021;59:1574-8. [Crossref] [PubMed]
- Venge P, van Lippen L, Blaschke S, et al. Equal clinical performance of a novel point-of-care cardiac troponin I (cTnI) assay with a commonly used high-sensitivity cTnI assay. Clin Chim Acta 2017;469:119-25. [Crossref] [PubMed]
- Braga F, Aloisio E, Panzeri A, et al. Analytical validation of a highly sensitive point-of-care system for cardiac troponin I determination. Clin Chem Lab Med 2019;58:138-45. [Crossref] [PubMed]
- Pickering JW, Young JM, George PM, et al. Validity of a Novel Point-of-Care Troponin Assay for Single-Test Rule-Out of Acute Myocardial Infarction. JAMA Cardiol 2018;3:1108-12. [Crossref] [PubMed]
- Gunsolus IL, Schulz K, Sandoval Y, et al. Diagnostic performance of a rapid, novel, whole blood, point of care high-sensitivity cardiac troponin I assay for myocardial infarction. Clin Biochem 2022;105-106:70-4. [Crossref] [PubMed]
- Apple FS, Smith SW, Greenslade JH, et al. Single High-Sensitivity Point-of-Care Whole-Blood Cardiac Troponin I Measurement to Rule Out Acute Myocardial Infarction at Low Risk. Circulation 2022;146:1918-29. [Crossref] [PubMed]
- Cullen L, Collinson PO, Giannitsis E. Point-of-care testing with high-sensitivity cardiac troponin assays: the challenges and opportunities. Emerg Med J 2022;39:861-6. [Crossref] [PubMed]
Cite this article as: Joyce LR, Pickering JW, Than M. Ruling out acute myocardial infarction based on a single high-sensitivity troponin measurement in the emergency department: a clinical practice review. J Lab Precis Med 2023;8:21.


