Humoral response is enhanced after ipsilateral double intramuscular injection of BNT162b2 COVID-19 vaccine
Highlight box
Key findings
• Double injection of coronavirus disease 2019 (COVID-19) vaccine into the ipsilateral arm elicits a stronger humoral immune response.
What is known and what is new?
• There are still unknown causes for the intensity and duration of immune response after COVID-19 vaccination.
• We found that COVID-19 vaccination elicits a stronger humoral response on the ipsilateral arm.
What is the implication, and what should change now?
• Administration of subsequent doses of COVID-19 vaccine to the ipsilateral arm may be preferable to vaccination to the contralateral arm.
Introduction
Although the World Health Organization (WHO) has decided to no longer consider coronavirus disease 2019 (COVID-19) a public health emergency of international concern as of March 2023 (1), there are several reasons for which vigilance against this potentially life-threatening infection should not be discontinued, foremost among them being the fact that relatively frequent, supposedly less severe, waves of increased infections have been observed due to the incessant spread of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants (2). Although most of these infections will result in only mild disease in most affected individuals because herd immunity (natural, vaccine-induced, or hybrid immunity) is now widespread, the more susceptible segments of the population, particularly immunocompromised and comorbid individuals, are at non-negligible risk of developing severe COVID-19 illness (3).
In this still rather unpredictable scenario, COVID-19 vaccines will remain the prime weapon in the fight against the future waves of the COVID-19 pandemic. It is now indisputable that vaccines are highly effective and have several key functions in reducing the clinical, social, and economic burden of COVID-19. These include lowering the risk of infection, reducing the risk of developing severe disease (with hospitalization, need for intensive care, and even death), helping to strengthen community immunity (herd immunity), promoting post-pandemic economic recovery, and enhancing public confidence and socialization (4).
Although the clinical utility of the vaccines in combating the still ongoing COVID-19 pandemic cannot be overstated, the intensity and duration of the immune response plays a key role in maintaining their efficacy (5). To date, many different types of COVID-19 vaccines have been developed (6); the most common vaccines messenger RNA (mRNA)-based vaccines, viral vector vaccines, protein subunit vaccines, and inactivated or attenuated live virus vaccines. Despite the different technology used for their manufacturing, their function is relatively similar. Basically, the vaccine is administered by intramuscular injection into the deltoid muscle, which is usually quick and relatively painless (to our knowledge, nasal vaccines are not yet on the market). With the exception of protein subunit vaccines, the vaccine particles are rapidly taken up by somatic or muscle cells and by tissue resident or recruited antigen-presenting cells after a variable period of time. The particles are also transported to local lymph nodes, where they come into contact with resident T and B cells, which are then responsible for generating a local immunological response (both humoral and cellular), which is then diffused to the rest of the body (7). With few exceptions, it may be necessary to inject multiple doses at short intervals to produce a sustained immunologic response. In the absence of anatomic or functional limitations, the choice of the arm in which to give the injections is typically left to the patient, who may decide to use the same arm each time or alternate one arm with the other for subsequent doses. However, because stimulation of immune cells in the same lymph nodes previously stimulated by the earlier dose(s) may potentially be more responsive to the subsequent boost, we planned a retrospective observational study to examine whether ipsilateral or contralateral administration of the two vaccine doses of the primary COVID-19 immunization cycle could elicit a different humoral response.
Methods
Study population
The study population consisted of a series of SARS-CoV-2 seronegative employees of the hospital of Peschiera del Garda in Italy who received the initial vaccination with the mRNA-based Comirnaty COVID-19 vaccine (Pfizer/Biontech, Mainz, Germany), consisting of two doses of 30 µg each, 3 weeks apart from each other. SARS-CoV-2 seronegativity meant that total Abs levels were <0.8 KBAU/L (kilo binding antibody units/L), as indicated below. Patients were free to choose in which arm to receive the first and second doses of vaccine, and this information was always recorded during vaccination. Blood was collected by venipuncture before receiving the first dose of vaccine (i.e., baseline), before the second dose of vaccine (21 days after the first dose), and then 1 month after the second dose (i.e., 51 days after the first dose). Humoral response was determined by measuring the serum concentration of total anti-SARS-CoV-2 antibodies (Abs) with the Roche Elecsys Anti-SARS-CoV-2 S chemiluminescence immunoassay on a Roche Cobas 6000 (Roche Diagnostics, Basel, Switzerland; positive result: ≥0.8 KBAU/L). This assay has an optimal correlation with SARS-CoV-2 neutralization potential developing after COVID-19 vaccination, as confirmed by a sensitivity of 98% compared with a pseudovirus neutralization assay (8). The analytical characteristics of this immunoassay were preliminarily investigated by Lippi et al. (9), who found an analytical imprecision comprised between 1.0–2.5%.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All subjects provided a written informed consent for participating to the study. This retrospective observational study was reviewed and approved by the Ethics Committee of the Provinces of Verona and Rovigo (No. 59COVIDCESC; November 8, 2021).
Statistical analysis
The ratio between total anti-SARS-CoV-2 Abs levels at 51 and 21 days was calculated. The results of all measurements were expressed as median and interquartile range (IQR). Statistical analysis was performed using Analyse-it (Analyse-it Software Ltd., Leeds, UK). The significance of differences was calculated using the Mann-Whitney U test.
Results
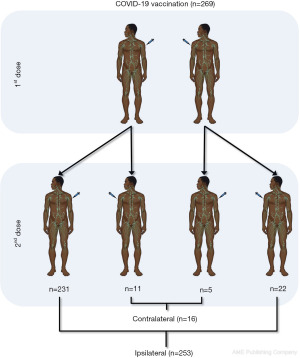
The final study population consisted in 269 ostensibly healthcare workers (median age, 44 years; IQR, 33–53 years; 109 men). Of these, 253 (94.1%) received both doses of vaccine on the deltoid of the same arm (i.e., ipsilateral), while 16 (5.9%) received the second dose on the deltoid of a different arm (i.e., contralateral). Of these 16 subjects, 11 chose the first dose for the left arm and the second dose for the right arm, while 5 chose the first dose for the right arm and the second dose for the left arm, as summarized in Figure 1. The demographic characteristics of the subjects are summarized in Table 1, which shows that no age or sex differences were observed between the two cohorts. Regarding total anti-SARS-CoV-2 Abs levels, no difference was found between the two groups both at baseline (i.e., <0.4 KBAU/L for all; P>0.99) or after the first vaccine dose (52 vs. 51 KBAU/L; P=0.243), whereas after the second dose a significantly higher level was found after ipsilateral vaccination compared to the contralaterally administered vaccines (1,437 vs. 1,052 KBAU/L; P=0.047) (Figure 2).
Table 1
| Variable | Second dose | P | |
|---|---|---|---|
| Contralateral (n=16) | Ipsilateral (n=253) | ||
| Age (years) | 43 [37–58] | 44 [33–53] | 0.261 |
| Sex (M/F) | 5/11 | 104/149 | 0.436 |
| Injection | 11 subject left arm and then right arm, 5 right arm and then left arm |
22 both right arm and 231 both left arm | – |
| Total anti-SARS-CoV-2 Abs (KBAU/L) | |||
| Baseline | All <0.4 | All <0.4 | >0.99 |
| 21 days after 1st dose | 51 [24–72] | 52 [17–106] | 0.243 |
| 30 days after 2nd dose | 1,052 [857–1,695] | 1,437 [838–2,293] | 0.047 |
| Ratio 2nd/1st dose | 25.2 [17.4–46.9] | 33.7 [18.3–62.9] | 0.007 |
Data were expressed as median [IQR] or n. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger RNA; COVID-19, coronavirus disease 2019; M, male; F, female; Abs, antibodies; KBAU, kilo binding antibody units; IQR, interquartile range.

Accordingly, the ratio of total anti-SARS-CoV-2 Abs levels after the second and first vaccine doses (i.e., 51/21 days) was significantly higher in ipsilaterally vaccinated patients than in those contralaterally vaccinated (33.7 vs. 25.2). In contrast, no significant difference was found in the ratio of the second-to-first dose of total anti-SARS-CoV-2 Abs between those who received the first vaccination on the left arm and the second dose on the right arm (26.2; IQR, 14.8–46.1, P=0.498) compared to those who received the first vaccine on the right arm and the second dose on the left arm (24.2; IQR, 21.2–44.3; P=0.498), nor between patients who received both vaccine doses on the right (34.0; IQR, 17.6–51.8) or left (33.7; IQR, 18.4–64.4; P=0.411) arms.
Discussion
The generation of a sustained and durable immunological response is the cornerstone of present and future strategies to address the still ongoing COVID-19 pandemic (10), the clinical and epidemiologic burden of which has now been attenuated but has by no means disappeared, especially following the emergence of new and potentially more virulent variants such as EG.5 (11) and BA.2.86 (12), both of which seem to exhibit increased transmissibility compared with earlier strains.
The mRNA vaccines have been widely used to combat the COVID-19 pandemic because of their many advantages, including high clinical efficacy against the risk of developing severe illness, simple design and adaptability of antigens, rapid and relatively inexpensive production, and convenient route of administration (i.e., usually intramuscular) (13). Regardless of the need for continuous updating of COVID-19 vaccine formulations, which occurs through the inclusion of sequences of newly emerged SARS-CoV-2 lineages, the ability to enhance the immune response is another essential aspect for reducing the likelihood of infection and, in particular, for providing enhanced immunologic protection to the more vulnerable segments of the population (i.e., patients with multiple and/or severe pathologies, the disabled and/or elderly, immunocompromised individuals) (14).
Administration of COVID-19 vaccines has been mainly intramuscular (into the deltoid muscle) because this site represents the best possible compromise between efficacy (in terms of immunologic response) and safety (in terms of side effects) (15). The decision as to which arm to vaccinate is usually left to the patient, who may choose one arm or the other for a variety of reasons, but may also switch arms from one vaccine dose to the next. Therefore, understanding whether ipsilateral or contralateral COVID-19 vaccine administration may be associated with a different immunologic response is an important consideration for optimizing vaccine efficacy depending on the route of delivery.
The results of our study demonstrate that mRNA-based Comirnaty COVID-19 may elicit a significantly different humoral response after ipsilateral or contralateral administration. Specifically, the increase in total anti-SARS-CoV-2 Abs after the second dose was 34% higher (33.7 vs. 25.2) in patients receiving dual ipsilateral vaccination than in those receiving dual contralateral administration. This is reflected in the fact that levels of these Abs were also 37% higher (1,437 vs. 1,052 KBAU/L) after the second dose in subjects who chose ipsilateral versus contralateral vaccination.
A similar reference can be found in the current scientific literature, as Ziegler et al. recently published the results of an observational study (16) in which 303 SARS-CoV-2 seronegative individuals were administered a primary cycle of the COVID-19 vaccine Comirnaty, and alternatively received the second dose of vaccine on the ipsilateral (n=147) or contralateral (n=156) arm. The authors reported that the levels of neutralizing SARS-CoV-2 Abs were significantly higher in those vaccinated ipsilaterally than in those vaccinated contralaterally (neutralizing activity: 69% vs. 65%; P=0.024). Of note, the authors did not measure total anti-SARS-CoV-2 Abs, but reported that anti-SARS-CoV-2 spike protein IgG did not differ after contralateral or ipsilateral vaccination (4,002 vs. 4,590 KBAU/L), whereas the rate of vaccinees with detectable anti-SARS-CoV-2 spike protein CD8 T cells was significantly higher after ipsilateral vaccination than after contralateral vaccine administration (67.2% vs. 43.0%; P=0.004).
There are some plausible biological aspects that could help explain our findings. The general population may respond better to successive vaccinations when administered on the ipsilateral arm because the vaccine particles directly target the same lymphoid structures as in the initial injection and these are more reactive and efficient in producing immune cells that can then perform their function (i.e., anti-SARS-CoV-2 neutralizing antibody or cytokine production, killing infected cells) more effectively. The initial priming followed by enhanced amplification of the immune response in the same draining axillary lymph nodes that occurs after ipsilateral injection is supported by several lines of evidence that consistently show that ipsilateral lymphadenopathy in the area of the injected deltoid muscle is a common consequence of COVID-19 vaccination (17,18). In a recent meta-analysis conducted by Co et al. (19), the overall frequency of clinically detectable axillary lymphadenopathy was estimated to be 0.4%, with a mean size of enlarged lymph nodes of approximately 18.2 mm (range, 16–21 mm), a mean duration of 7 days (range, 2–18 days), and typical resolution within 1 month. However, clinical evidence of COVID-19 vaccine-induced lymphadenopathy cannot be considered a benchmark because it cannot reliably detect minor changes in lymph node structure, biology, and metabolism that may have occurred after immunostimulation.
In a comprehensive study, Yoshikawa et al. used magnetic resonance imaging (MRI) for obtaining pre- and post-vaccination chest scans in 433 subjects who had undergone a primary vaccination cycle (i.e., two doses) with Pfizer-BioNTech or Moderna COVID-19 vaccines (20). Of note, the cumulative incidence of axillary lymphadenopathy was as high as 21% up to 2 weeks after vaccination. The prevalence of hypermetabolic lymph nodes detected by FDG PET/CT (F-fluorodeoxyglucose positron emission tomography/computed tomography) was estimated in the previous meta-analysis published by Treglia et al. (21). The authors selected nine published articles with a total of 2,354 COVID-19 patients in whom vaccine recipients underwent FDG PET/CT examination. The cumulative incidence of hypermetabolic axillary lymph nodes ipsilateral to the vaccine injection site was 37% [95% confidence interval (CI): 27–47%]. Bernstine et al. also conducted a retrospective cohort study (22), obtaining FDG PET/CT scans of 650 consecutive patients who underwent BNT162b2 vaccination. Ipsilateral hypermetabolic axillary lymph nodes were observed in 14.5% and 43.3% of subjects after the first and second vaccine doses, respectively. In a more recent study, Calabria et al. also used FDG PET/CT scans to examine 104 patients undergoing mRNA-based COVID-19 vaccination (23) and reported that ipsilateral axillary and/or deltoid tracer uptake was present in up to 85% of all vaccinees.
Thus, a strong immune response to the vaccine may well trigger the development of COVID-19 vaccine-associated lymphadenopathy, and as recently pointed out by Ho et al., the underlying cause could be a sustained B-cell germinal center response after vaccination (24).
Conclusions
Taken together, previous evidence would hence support the conclusion of our retrospective observational study that ipsilateral COVID-19 vaccination may be associated with a stronger immune response than contralateral vaccination. With this in mind, we proffer that the use of the same arm for COVID-19 vaccine injection may be preferable, especially for inherently vulnerable individuals who will need a better immunologic protection against COVID-19 and its continuously emerging variants.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-56/dss
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-56/coif). GL serves as the Editor-in-Chief of Journal of Laboratory and Precision Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All subjects provided a written informed consent for participating to the study. This retrospective observational study was reviewed and approved by the Ethics Committee of the Provinces of Verona and Rovigo (No. 59COVIDCESC; November 8, 2021).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wise J. Covid-19: WHO declares end of global health emergency. BMJ 2023;381:1041. [Crossref] [PubMed]
- Callaway E. COVID's future: mini-waves rather than seasonal surges. Nature 2023;617:229-30. [Crossref] [PubMed]
- Lippi G, Plebani M. COVID-19: the global health emergency is over for the WHO, but not yet for laboratory medicine. J Lab Precis Med 2023;8:17. [Crossref]
- Dye C. The benefits of large scale covid-19 vaccination. BMJ 2022;377:o867. [Crossref] [PubMed]
- Lippi G, Henry BM, Plebani M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics (Basel) 2021;11:941. [Crossref] [PubMed]
- Kudlay D, Svistunov A, Satyshev O. COVID-19 Vaccines: An Updated Overview of Different Platforms. Bioengineering (Basel) 2022;9:714. [Crossref] [PubMed]
- Callaway E. The race for coronavirus vaccines: a graphical guide. Nature 2020;580:576-7. [Crossref] [PubMed]
- Douxfils J, Gillot C, Mullier F, et al. Post-SARS-CoV-2 vaccination specific antibody decrease - Thresholds for determining seroprevalence and seroneutralization differ. J Infect 2021;83:e4-5. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Pegoraro M, et al. Preliminary evaluation of Roche Cobas Elecsys Anti-SARS-CoV-2 chemiluminescence immunoassay. Clin Chem Lab Med 2020;58:e251-3. [Crossref] [PubMed]
- Lippi G, Henry BM, Plebani M. Optimizing effectiveness of COVID-19 vaccination: will laboratory stewardship play a role? Clin Chem Lab Med 2021;59:1885-8. [Crossref] [PubMed]
- Abbasi J. What to Know About EG.5, the Latest SARS-CoV-2 "Variant of Interest". JAMA 2023;330:900-1. [Crossref] [PubMed]
- Looi MK. Covid-19: Scientists sound alarm over new BA.2.86 "Pirola" variant. BMJ 2023;382:1964. [Crossref] [PubMed]
- Zhou W, Jiang L, Liao S, et al. Vaccines’ New Era-RNA Vaccine. Viruses 2023;15:1760. [Crossref] [PubMed]
- Couzin-Frankel J. Decision nears for fall coronavirus vaccine. Science 2023;380:784. [Crossref] [PubMed]
- Thomas KS. Intramuscular Injections for COVID-19 Vaccinations. J Nucl Med Technol 2021;49:11-2. [Crossref] [PubMed]
- Ziegler L, Klemis V, Schmidt T, et al. Differences in SARS-CoV-2 specific humoral and cellular immune responses after contralateral and ipsilateral COVID-19 vaccination. EBioMedicine 2023;95:104743. [Crossref] [PubMed]
- Tu W, Gierada DS, Joe BN. COVID-19 Vaccination-Related Lymphadenopathy: What To Be Aware Of. Radiol Imaging Cancer 2021;3:e210038. [Crossref] [PubMed]
- Turan A, Kaplanoğlu H, Kaplanoğlu V. Frequency of Ipsilateral Axillary Lymphadenopathy After the Inactivated COVID-19 Vaccine. Curr Med Imaging 2022;18:1214-21. [Crossref] [PubMed]
- Co M, Wong PCP, Kwong A. COVID-19 vaccine associated axillary lymphadenopathy - A systematic review. Cancer Treat Res Commun 2022;31:100546. [Crossref] [PubMed]
- Yoshikawa T, Miki S, Nakao T, et al. Axillary Lymphadenopathy after Pfizer-BioNTech and Moderna COVID-19 Vaccination: MRI Evaluation. Radiology 2023;306:270-8. [Crossref] [PubMed]
- Treglia G, Cuzzocrea M, Giovanella L, et al. Prevalence and Significance of Hypermetabolic Lymph Nodes Detected by 2-[18F]FDG PET/CT after COVID-19 Vaccination: A Systematic Review and a Meta-Analysis. Pharmaceuticals (Basel) 2021;14:762. [Crossref] [PubMed]
- Bernstine H, Priss M, Anati T, et al. Axillary Lymph Nodes Hypermetabolism After BNT162b2 mRNA COVID-19 Vaccination in Cancer Patients Undergoing 18F-FDG PET/CT: A Cohort Study. Clin Nucl Med 2021;46:396-401. [Crossref] [PubMed]
- Calabria F, Bagnato A, Guadagnino G, et al. COVID-19 vaccine related hypermetabolic lymph nodes on PET/CT: Implications of inflammatory findings in cancer imaging. Oncol Res 2023;31:117-24. [Crossref] [PubMed]
- Ho TC, Shen DH, Chang CC, et al. Immune Response Related to Lymphadenopathy Post COVID-19 Vaccination. Vaccines (Basel) 2023;11:696. [Crossref] [PubMed]
Cite this article as: Pighi L, Henry BM, Salvagno GL, Lippi G. Humoral response is enhanced after ipsilateral double intramuscular injection of BNT162b2 COVID-19 vaccine. J Lab Precis Med 2023;8:28.