Identification of interferences in free thyroxin assay—establishment of acceptable recovery limits for the dilution test
Interferences due to endogenous immunoglobulins could cause clinically misleading results in immunoassays. Different methods can be used to verify the possible presence of interferences in results that conflict with the clinical findings (1-3). One of the simplest and most effective tests is the dilution test (4). However, in the automated immunoassays for free T4 (fT4) determination the dilution is generally not recommended since the sample diluent can often interfere with the free/bound equilibrium, giving ambiguous results (5).
The law of mass action states that, as a rough approximation, the free hormone concentration should remain constant if the ratio between bound hormone and serum binding capacity does not change (6). Then, dilutions of a serum with an inert buffer should give constant concentrations of free hormone. This method was used to verify the serum binding capacity bias of different assays (7,8), often showing unsatisfactory results. However, Oostendorp and Lentjes (9) found that a dilution test with saline solution could be used with Access Free T4 assay from Beckman Coulter Inc. (Brea, CA, USA) as a possible method for detecting interference in the assay of free thyroxine. The aim of this study was to confirm the data previously published with the Access fT4 method and to determine an acceptable range for recovery, beyond which the presence of interference can be considered probable in cases with incongruous laboratory data.
For the preliminary evaluation of the dilution test, three groups of subjects were selected: four patients admitted to the intensive care unit for infectious disease or cardiac failure, five pregnant women between the second and third trimesters and four healthy subjects.
A dilution series ranging from 2 to 40 times was done for all the samples both in saline (Monico s.p.a, Venice, Italy) and in the inert buffer Tris(hydroxymethyl)-aminomethane (TRIS) buffer solution, pH 7.4 (Carlo Erba, Milan, Italy).
To determine an acceptable recovery range of the dilution test, the method of reference interval (RI) determination was used. One hundred twenty-one cases (63 women, 58 men, median age 53 years, age range 19–98 years) were then selected and dilutions 1/5 evaluated according to the CLSI guideline EP28A3c (10). The non-parametric method was used, without data transformations. The Tukey test was used for outliers’ identification. No age or sex-related differences in recovery were found (data not shown).
Serums collected from routine samples were measured on the same day or kept frozen (−20 ℃) until the time of analysis. Given the aim of the study, samples were chosen to represent “real world” circumstances and to obtain acceptable ranges of the dilutions in the most diverse conditions. Therefore, cases with values both within and outside the RI were included, as long as there aren’t interferences.
Samples were selected based on the congruence of the results of fT3, fT4 and thyroid stimulating hormone (TSH). For samples with results outside their respective RI, in addition to laboratory data, the selection was also based on the clinical plausibility of the result. Moreover, intensive care unit patients, pregnant women, patients from cardiology departments, psychiatric patients and patients with renal dysfunction were excluded.
A total of 84 samples were from euthyroid subjects, 26 samples were hyperthyroid subjects (mainly Graves’ disease with the need for increased therapy, hyperthyroidism at the onset, acute thyroiditis. There was one case of polymorphism of deiodinase). Eleven samples were from hypothyroid subjects, with one case of hypothyroidism after iodotherapy.
In addition, 44 cases were evaluated with inconsistent results among the measured thyroid hormones. This discrepancy at the time of case selection was not justified by a particular pathophysiological condition, or by drugs known to alter the concentration of free thyroxine and/or TSH.
fT4 was measured by the automated Access Free T4 assay (Beckman Coulter Inc.), a competitive two-stage chemiluminescence assay using paramagnetic particles performed by the platform UniCel DxI 800 according to the manufacturer’s instructions. The amount of analyte in the sample was determined by means of a multi-point calibration. The RI was 7.9–14.7 nmol/L (11).
To verify the congruence of the data, all samples were also determined on a second analytical platform, the competitive chemiluminescence single-step method with magnetic microparticles coated with T4 analogue, performed by Liaison XL platform (DiaSorin S.p.A., Saluggia, Italy). The RI was 10.3–22 nmol/L, as proposed by the manufacturer. The presence of heterophilic antibodies was evaluated using Heterophilic Blocking Tubes (HBT, Scantibodies Laboratory Inc., Santee, CA, USA) according to the manufacturer’s instructions.
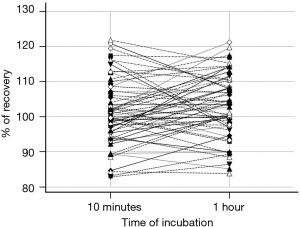
The samples were measured on DxI 800 both undiluted and after 1/5 dilution with saline (1 part sample + 4 parts saline) and short incubation of 10 minutes. To exclude a possible influence of the incubation time, 61 samples were also measured with 1/5 dilution after 1 hour of incubation.
The means of the determinations with TRIS buffer and saline were compared by t-test. The results of the dilutions after different incubation time were compared by Wilcoxon test. The statistical significance was set at P<0.05. Statistical analysis was performed with MedCalc © Software, Version 7.4.2.0 (MedCalc Software, Mariakerke, Belgium).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of ULSS 3 Serenissima (approval No. 149/ACESC) and individual consent for this retrospective analysis was waived.
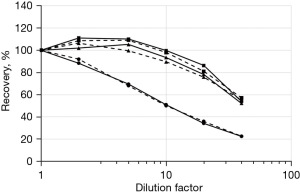
The results of the dilution tests in the three groups of subjects (pregnant, critically ill and control subjects) are shown in Figure 1. Both in controls and in pregnant women, an excellent linearity is shown up to at least 1/10 dilution. In critically ill patients the recovery dropped by more than 30% already at a 1/5 dilution. Since no statistically significant difference between TRIS buffer dilution and saline was found (t-tests P>0.1), the subsequent evaluations were performed using saline solution, as in Oostendorp and Lentjes (9). The 1/5 dilution, sufficient in most cases to reduce any interference and less disturbing the free-total hormone equilibrium, was chosen.
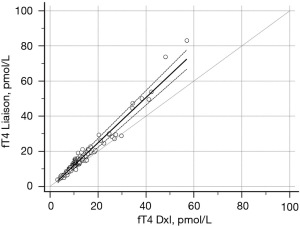
In the 121 samples selected for determination of an acceptable recovery after dilution, the correlation between the DxI method and Liaison was excellent, with an expected overestimation of the Liaison method, according to the different RI between the two methods, but without clearly aberrant results. With the Passing-Bablock method the regression equation “Liaison = 0.94 (−1.8/+0.05) + 1.29 (1.2/1.36) DxI” was obtained (Figure 2). This further confirms that the results do not reasonably present any kind of interference.
The results of the 61 cases measured after 1-hour incubation did not show differences statistically significant from those after a 10-minute incubation (Wilcoxon test for paired data P=0.10; Figure 3).
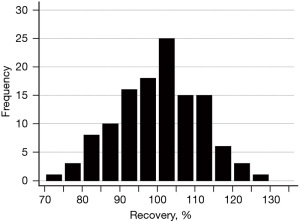
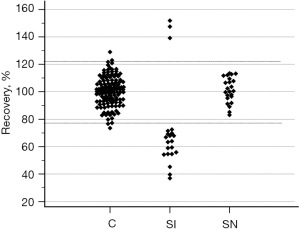
The results of dilution 1/5 recoveries in the 121 control subjects are shown in Figure 4. No outliers were found. The acceptable range (2.5th–97.5th percentile) of recovery was between 77.4% [90% confidence interval (CI): 73.4–83%] and 121.7% (90% CI: 116.5–129%).
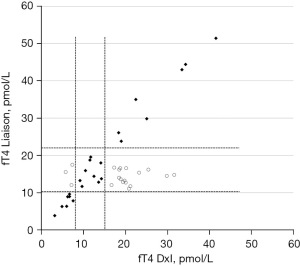
In 23 out of 44 cases evaluated for possible interference, results of the 1/5 dilution showed recoveries within the established range of acceptability, and fT4 values on the Liaison XL platform in the undiluted samples were in qualitative agreement (within or outside the respective RI) with the results on DxI platform (Figure 5). However, in 21 cases, recoveries were outside the acceptable range. In the latter cases, the concentrations of the undiluted samples measured on Liaison XL were instead markedly different from DxI 800 (Figure 5), and in accordance with those found for TSH. In particular, they were outside the RI with DxI and within RI with Liaison XL. In subjects with probable interference, only 7 showed significant variation in fT4 after incubation with HBT, suggesting the possible presence of interfering heterophilic antibodies. In two other cases the inconsistencies detected by dilution were due to the intake of high levels of biotin by patients. In these cases, it was not necessary to compare the data on a second analytical platform. A new sampling was carried out a few days after the interruption of the vitamin intake and showed the normalization of the fT4 values (12).
Comparisons of recoveries in the three different classes of subjects considered (controls, suspected cases with interference, suspected cases without interference) in relation to the acceptable ranges were illustrated in Figure 6.
In the measurement of free thyroid hormones, in agreement with the law of mass action, a constant measurement should be obtained after serial dilution of the sample. However, this occurs with reference methods such as equilibrium dialysis, but not always with immunoassay methods on automated platforms (7,8,13,14).
The dilution tests carried out in the present study show that the DxI method is sufficiently robust to sample dilution. In cases with high capacity of binding proteins, but also in more physiological situations, the linearity of the response remains optimal at least up to the factor 1/10. Only in conditions of poor protein binding capacity, as in critically ill patients (15), recovery already drops significantly since 1/5 dilution.
The use of a dilution test to detect the presence of possible interference is a widely used methodology, but it is generally not recommended in the direct measure of free hormones (5). However, the good response to the dilution of the DxI platform, highlighted in the present study, allowed Oostendorp and Lentjes (9) to propose this method in the evaluation of fT4, showing a significant difference in the dilution results between subjects with and without interference. Moreover, in our study we determined an acceptable interval of dilution recovery on 121 subjects without interference, using a methodology for the determination of any other reference range. These subjects were identified based on clinical and laboratory criteria and confirmed by the assay on another analytical platform. We then evaluated 44 cases that showed results with incongruity among the analytes related to thyroid function. In 23 of these, dilution recovery remained within the identified acceptable range, and the determination on Liaison XL in the undiluted samples confirmed the fT4 result. In general, it is very unlikely that a type of interference will alter the measurement in two methods on two different instruments, with different test architecture and based on two different measurement principles. Moreover, in several of these cases a possible explanation for the apparent incongruity of the results has emerged. For example, one case was related to a patient bedridden due to a chronic pathology and possible affected by a euthyroid sick syndrome. Another was a case of hyperthyroidism at the beginning of therapy (with fT4 lowered but TSH still below RI). In other cases, a central hypothyroidism in patients with a previous brain tumor, and a possible polymorphism of deiodinase were found.
However, for 21 samples the recovery was completely outside the acceptable range, and in all of them the fT4 determination with Liaison XL showed results within the RI, except for the two cases with biotin interference and in which the evaluation with the Liaison method was not necessary.
For seven of the interfered samples, there was a possible interference from heterophilic antibodies, since the fT4 results were also normalized after the incubation with HBT. Two other interferences were due to a biotin supplementation, as mentioned above. In others, the source of the interference remains substantially unknown. This could be caused by heterophilic antibodies not detected by HBT or by other “natural” low affinity antibodies, e.g., directed against the analyte or the reagents of the test (16).
A possible limitation of this study may be the impossibility of establishing a real predictive value of the test: although all the interfered samples have been correctly detected, the suspected cases, both with and without interferences, have not been collected consecutively and do not represent the respective frequency with which such cases occur.
In addition, even these reference ranges should not be interpreted too rigorously but must be evaluated on a case-by-case basis. For example, samples with high concentrations of fT4 could show lower recoveries even in absence of interferences. Moreover, patients in critical clinical conditions always show an excessively low recovery, and the acceptable limit determined in this study cannot be considered suitable.
In conclusion, we could verify that the method used has in general a relatively low bias due to serum binding capacity and allows to obtain an excellent recovery at the dilution test, at least up to the factor 1/10. The reference ranges established for the acceptable recovery under non-interfered conditions allow to accurately identify a possible analytical interference, with a simple and safe test applicable in the majority of routine cases.
Acknowledgments
We thank Dr. Antonette E. Leon (Regional Center for Biomarkers, Department of Clinical Pathology, Azienda ULSS 3 Serenissima, Venice, Italy) for her contribution in the revision of the English language.
Funding: None.
Footnote
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ismail AA. A radical approach is needed to eliminate interference from endogenous antibodies in immunoassays. Clin Chem 2005;51:25-6. [Crossref] [PubMed]
- Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev 2004;25:105-20. [PubMed]
- Wauthier L, Plebani M, Favresse J. Interferences in immunoassays: review and practical algorithm. Clin Chem Lab Med 2022;60:808-20. [Crossref] [PubMed]
- Ghazal K, Brabant S, Prie D, et al. Hormone Immunoassay Interference: A 2021 Update. Ann Lab Med 2022;42:3-23. [Crossref] [PubMed]
- Faix JD. Principles and pitfalls of free hormone measurements. Best Pract Res Clin Endocrinol Metab 2013;27:631-45. [Crossref] [PubMed]
- Ekins R. The free hormone hypothesis and measurement of free hormones. Clin Chem 1992;38:1289-93. [Crossref] [PubMed]
- Sapin R. Serum thyroxine binding capacity-dependent bias in five free thyroxine immunoassays: assessment with serum dilution experiments and impact on diagnostic performance. Clin Biochem 2001;34:367-71. [Crossref] [PubMed]
- Christofides ND, Wilkinson E, Stoddart M, et al. Assessment of serum thyroxine binding capacity-dependent biases in free thyroxine assays. Clin Chem 1999;45:520-5. [Crossref] [PubMed]
- Oostendorp M, Lentjes EG. Utility of dilution tests in investigating interference in the free thyroxine assay. Clin Chem Lab Med 2017;55:e4-6. [Crossref] [PubMed]
- CLSI and IFCC. EP28-A3 document; defining, establishing and verifying reference intervals in the clinical laboratory: approved guideline. 3rd ed. Wayne, PA: CLSI; 2010;28:1-76.
- Dittadi R, Carraro P. Age- and sex-related reference interval for free thyroxine: An indirect approach. Ann Clin Biochem 2021;58:675-7. [Crossref] [PubMed]
- Batista MC, Ferreira CES, Faulhaber ACL, et al. Biotin interference in immunoassays mimicking subclinical Graves' disease and hyperestrogenism: a case series. Clin Chem Lab Med 2017;55:e99-103. [Crossref] [PubMed]
- Nelson JC, Weiss RM, Wilcox RB. Underestimates of serum free thyroxine (T4) concentrations by free T4 immunoassays. J Clin Endocrinol Metab 1994;79:76-9. [PubMed]
- Favresse J, Burlacu MC, Maiter D, et al. Interferences With Thyroid Function Immunoassays: Clinical Implications and Detection Algorithm. Endocr Rev 2018;39:830-50. [Crossref] [PubMed]
- Nelson JC, Wilcox RB. Analytical performance of free and total thyroxine assays. Clin Chem 1996;42:146-54. [Crossref] [PubMed]
- Levinson SS, Miller JJ. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin Chim Acta 2002;325:1-15. [Crossref] [PubMed]
Cite this article as: Dittadi R, Lombino S, Carraro P. Identification of interferences in free thyroxin assay—establishment of acceptable recovery limits for the dilution test. J Lab Precis Med 2023;8:30.