Woman with cryptic chronic back pain: a case report
Highlight box
Key findings
• Non-secretory multiple myeloma (NSMM) is an uncommon subtype of myeloma characterized by negative serum protein electrophoresis (SPEP), urine protein electrophoresis (UPEP) and serum free light chain (sFLC).
What is known and what is new?
• Absence of monoclonal immunoglobulin in screening SPEP and sFLC does not rule out myeloma entirely if clinical suspicion is high. Absence of monoclonal immunoglobulin may reduce clinical features, defining events and end-organ damage typical of myeloma.
• Monitoring of NSMM patients relies on bone marrow assessment and positron emission tomography-computed tomography (PET-CT) examinations.
What is the implication, and what should change now?
• The current International Myeloma Working Group (IMWG) definition for NSMM does not include sFLC and may lead to inaccurate diagnosis, hence an update or review is needed.
Introduction
Non-secretory multiple myeloma (NSMM) was first described in 1958 (1). It is under-appreciated due to its much lower prevalence compared to the secretory myeloma. The underlying mechanism for the inhibition of the production or secretion of the monoclonal immunoglobulins by the clonal plasma cells are not fully understood. Several potential causes have been postulated, including loss of the polyadenylation site necessary for extracellular immunoglobulin secretion, loss of the heavy chain V domain leading to decreased secretion and increased destruction intracellularly and mutation of the immunoglobulin light chain (2).
NSMM may manifest as either the incapability of malignant plasma cells to produce immunoglobulin (non-producing), characterized by the absence of cytoplasmic immunoglobulin synthesis, or as an inability to discharge heavy or light chains (non-secretory) (3). In a clinical review of 197 NSMM patients, 19 patients (~10%) were found to have no cytoplasmic immunoglobulin by flow cytometry classifying them as the non-producer variant of NSMM with the rest being the non-secretor variant (4).
The current definition of NSMM by the International Myeloma Working Group (IMWG) is a myeloma characterised by absence of monoclonal immunoglobulins detected by serum protein electrophoresis (SPEP) and/or urine protein electrophoresis (UPEP). This definition does not consider the findings of serum free light chain (sFLC) assay, which has a lower analytical sensitivity compared to the electrophoresis methods. Additionally, this definition may erroneously exclude light chain-secreting only myeloma that is detected by sFLC. The current definition may require an update to incorporate advances in laboratory techniques (5).
The NSMM is distinguished from oligosecretory myeloma by the latter having detectable monoclonal immunoglobulin component that is below the threshold of a measurable disease. This measurable disease is defined as serum monoclonal immunoglobulin of ≥10 g/L, monoclonal immunoglobulin excretion in urine of >200 mg/24 hours or ≥100 mg/L of involved light chain with an associated abnormal kappa:lambda ratio (6).
On the other hand, the secretory form of myeloma clinical manifestations was caused by invasive growth of malignant cells, primarily in the bone and bone marrow, and from the production of monoclonal immunoglobulin. Symptoms related to the former include bone pain, osteolytic lesions, hypercalcaemia, and cytopenias. Conversely, the production of monoclonal immunoglobulin can lead to a variety of issues, including neuropathy and renal injury, which can occur through various mechanisms. We present this case in accordance with the CARE reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-33/rc).
Case presentation
A 61-year-old woman without past medical or family history of malignant diseases presented to the outpatient department complaining of persistent lower back pain for the past 2 months without any history of trauma or fall. She was otherwise well and was diagnosed with lower back soft tissue injury and treated with analgesic. Two weeks later, the patient visited the emergency department with worsening back pain and difficulty walking and performing activities of daily living. She was admitted and laboratory (Table 1) and radiological investigations were performed.
Table 1
| Parameters | Results | Reference values |
|---|---|---|
| Laboratory results | ||
| Hemoglobin (g/dL) | 11.3 | 11.2–15.7 |
| White blood cells (×103/μL) | 9.8 | 3.7–10.3 |
| Neutrophil (×103/μL) | 3.3 | 1.6–6.1 |
| Lymphocytes (×103/μL) | 2.2 | 1.2–3.9 |
| Red blood cells (×106/μL) | 4.10 | 3.90–5.20 |
| MCV (fL) | 93 | 79–98 |
| MCH (pg) | 28 | 26.0–32.0 |
| Platelet (×103/μL) | 180 | 155–369 |
| Urea (mmol/L) | 3.1 | 2.8–7.2 |
| Sodium (mmol/L) | 141 | 132–146 |
| Potassium (mmol/L) | 3.8 | 3.5–5.1 |
| Chloride (mmol/L) | 102 | 98–107 |
| Creatinine (μmol/L) | 48 | 45–84 |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | >90 | – |
| Calcium (mmol/L) | 2.66 | 2.20–2.65 |
| Magnesium (mmol/L) | 0.82 | 0.77–1.03 |
| Phosphate (mmol/L) | 0.79 | 0.81–1.45 |
| Total protein (g/L) | 74 | 64–83 |
| Albumin (g/L) | 40 | 34–48 |
| Albumin:globulin ratio | 1.18 | – |
| Aspartate aminotransferase (U/L) | 36 | <35 |
| Alanine aminotransaminase (U/L) | 18 | 5–55 |
| Total bilirubin (μmol/L) | 8 | 5–21 |
| Direct bilirubin (μmol/L) | 2.1 | <3.4 |
| Lactate dehydrogenase (U/L) | 237 | 125–220 |
| Alkaline phosphatase (U/L) | 92 | 40–150 |
| C-reactive protein (mg/L) | 5.0 | 0.4–4.9 |
| Glucose (mmol/L) | 6.5 | <7.8 |
| Serum protein electrophoresis and immunofixation | ||
| Total protein (g/L) | 74 | – |
| Albumin (g/L) | 42.5 | 38.3–60.1 |
| Alpha-1 globulin (g/L) | 0.9 | 0.6–2.7 |
| Alpha-2 globulin (g/L) | 10.6 | 4.7–10.5 |
| Beta globulin (g/L) | 11.0 | 4.8–10.7 |
| Gamma globulin (g/L) | 9.0 | 5.1–13.1 |
| Total IgG (g/L) | 7.3 | 5.5–16.3 |
| Total IgA (g/L) | 0.8 | 0.7–5.2 |
| Total IgM (g/L) | 0.4 | 0.3–2.9 |
| sFLC analysis (Freelite assay on Optilite analyser) | ||
| sFLC, kappa (mg/L) | 8.94 | 3.30–19.40 |
| sFLC, lambda (mg/L) | 7.11 | 5.71–26.30 |
| sFLC ratio | 1.26 | 0.26–1.65 |
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; eGFR, estimated glomerulus filtration rate; CKD-EPI, chronic kidney disease epidemiology collaboration equation; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; sFLC, serum free light chain.
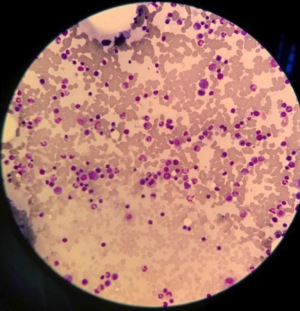
Her whole blood cell count performed on Sysmex XN platform (Sysmex corporation, Kobe, Japan) was normal, and her basic serum biochemistry investigations performed on Abbott Alinity (Abbott laboratories, IL, USA) platform revealed mild hypercalcaemia of 2.66 mmol/L (reference interval: 2.20–2.65 mmol/L) and was otherwise unremarkable. Her peripheral blood smear showed presence of occasional plasma cells with no rouleaux formation. Her SPEP and UPEP did not reveal any restricted band suggestive of monoclonal immunoglobulin (Figure 1). There was alpha-2 and beta hyperglobulinaemia that were suggestive of an underlying inflammatory process. Her kappa and lambda free light chain was 8.94 and 7.11 mg/L, respectively with sFLC ratio of 1.26 and were within normal range. Her plain X-ray showed multiple lytic lesions over the lumbar region with compression at L3–L5 level.
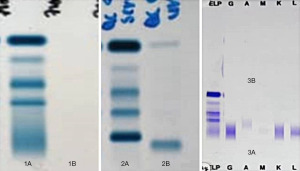
In view of the clinical, radiological and peripheral blood picture findings that were suggestive of multiple myeloma, serum immunofixation electrophoresis (sIFE) and urine immunofixation electrophoresis (uIFE) were performed following discussion with the attending clinician, which returned negative. She subsequently underwent bone marrow and trephine biopsy with flow cytometry study (Figure 2). The bone marrow biopsy myelogram revealed 30% medullary plasmocytosis with immunohistochemistry and flow cytometry showed kappa restriction and CD138-positive findings.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was unable to be obtained from the patient for publication of this case report and accompanying images due to patient’s relocation.
Discussion
This patient had >10% clonal bone marrow plasma cells with raised serum calcium which is part of the calcium elevation, renal dysfunction, anaemia and bone disease (CRAB) feature. Although her serum calcium was raised, it did not reach the diagnostic limit of >2.75 or 0.25 mmol/L higher than upper reference limit. The absence of detectable monoclonal immunoglobulin component on serum and UPEP as well as normal sFLC measurements meets diagnostic criteria for NSMM.
The primary complaint of this patient was back pain and was most likely due to the lytic bone lesion. Her biochemical changes may be attributed to it as well. Her hypercalcaemia, albeit mild, may be secondary to the release of calcium from the bone matrix through increased bone resorption. The serum phosphorus concentration just below the reference interval may be due to the formation of insoluble calcium-phosphate complexes in the bone matrix. Other serum biochemistry changes in lytic bone changes include elevation in aspartate aminotransferase and lactate dehydrogenase that are released from damaged bone tissue and are marginally elevated in this patient. Alkaline phosphatase may also be increased secondary to enhanced bone turnover and the release of alkaline phosphatase from osteoblasts during the bone remodeling process.
Her renal function, as assessed by estimated glomerular filtration rate, and hemoglobin were within reference intervals, and did not fulfil the renal end organ damage and anaemia diagnostic criteria. The causes of renal insufficiency in myeloma are often due to presence of light chains in the kidney, which is directly nephrotoxic to the proximal renal tubules. Additionally, it may accumulate and precipitate, forming cast in the distal tubules leading to renal obstruction (7). The absence of detectable monoclonal immunoglobulin in the serum and urine of this patient may explain the preserved renal function. Nevertheless, the most common pathophysiological mechanism of anaemia in myeloma is due to chronic disease, erythropoietin deficiency secondary to renal insufficiency and side effects of myelosuppressive chemotherapeutic agents (8). Compared to patients with secretory myeloma, patients with oligosecretory myeloma less often have renal dysfunction and hypercalcaemia.
Besides the absence of monoclonal immunoglobulin in the SPEP that was corroborated by UPEP and IFE, there was an increase in alpha-2 and beta globulin fractions. Alpha-2 globulin can increase in lytic bone changes due to the release of alpha-2 macroglobulin from osteoclasts during bone resorption. Alpha-2-macroglobulin is a protease inhibitor that is produced by a variety of cells, including osteoclasts, and is involved in the regulation of bone turnover. Increased expression of beta globulin like beta-2 microglobulin may be seen on the surface of myeloma cells and may indicate more advanced disease. Elevated C-reactive protein is also seen in active myeloma disease.
The recommended standard workup for patients who are either diagnosed with or suspected of having NSMM, as outlined in the consensus statement from the International Myeloma Workshop [2003], includes SPEP, UPEP and sFLC testing. Additionally, an imaging survey is recommended as part of the evaluation process. For all patients with suspected myeloma, including NSMM, a bone marrow aspiration (or biopsy of suspected plasmacytomas) should be performed. This procedure is complemented by flow cytometry and CD138-enriched fluorescent in situ hybridization testing. If true NSMM is suspected, samples should also be stained for intracellular immunoglobulin. Radiological investigations include positron emission tomography-computed tomography (PET-CT) scan. Like other forms of symptomatic myeloma, the diagnosis of NSMM requires the presence of myeloma-defining events and/or evidence of myeloma-mediated end-organ damage, such as hypercalcaemia, anaemia, or bone lesions. This differentiation is crucial in distinguishing between an asymptomatic myeloma precursor and a malignant myeloma (9).
Monitoring of NSMM is a challenge owing to its non-secretory nature. Standard monitoring modalities such as SPEP, UPEP and sFLC quantitation are uninformative. For these patients, bone marrow biopsies should be repeated 3–6 monthly during induction treatment, together with whole-body PET-CT examination 6–12 weekly. For NSMM patients who achieve remission following treatment or during maintenance therapy, repeat bone marrow biopsy and PET-CT examination 3–6 monthly is indicated. This regular monitoring helps assess the response to treatment and detect any potential relapse or disease progression. Nevertheless, a case report described a NSMM transforming to secretory type during its relapse after initial resolution of all active focal lesion and decreasing bone marrow plasmacytosis. In such scenario, SPEP, UPEP and sFLC will become relevant investigations (10).
While mass spectrometry (MS) methodologies were not mentioned in the 2003 IMWG recommendation for NSMM standard work-up, they are more sensitive analytically in paraprotein detection in serum samples. This is illustrated in a study by Giles et al. (11) whereby previously classified NSMM patients based on SPEP, SIFE and sFLC were evaluated for detectable paraprotein using MS. Among the 22 patients in the study cohort, 20 (91%) of them had detectable paraprotein. Although MS is not widely available at the moment, but findings from this study may indicate that many previously classified NSMM patients does have low level paraprotein secretion.
While NSMM may appear to have better prognosis than secretory multiple myeloma possibly due to the lack of pathological effect of secreted monoclonal immunoglobulins, this hypothesis has not been confirmed. The scarce NSMM data and participation in clinical trials impedes accurate assessment of its prognosis. In a retrospective study examining the survival and prognosis of NSMM patients diagnosed between 2001 and 2012 and treated with conventional chemotherapy, high dose chemotherapy and autologous stem cell transplant, the overall survival of NSMM was superior compared to secretory myeloma with a median of 8.3 vs. 5.4 years (P=0.03) (5).
Conclusions
SPEP and immunofixation together with sFLC are recommended screening tests for suspected case of multiple myeloma. A negative screening test panel should not be solely used to exclude the diagnosis if the clinical suspicion is high as illustrated in this case.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-33/rc
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-33/coif). TPL serves as an unpaid Associate Editor-in-Chief of Journal of Laboratory and Precision Medicine from July 2021 to December 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was unable to be obtained from the patient for publication of this case report and accompanying images due to patient’s relocation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gray ST, Antunovic DM, White AE. Non secretory multiple myeloma involving the maxilla: report of a case with update of biology and new approaches to management. Oral Oncol 1997;33:136-40. [Crossref] [PubMed]
- Cogné M, Guglielmi P. Exon skipping without splice site mutation accounting for abnormal immunoglobulin chains in nonsecretory human myeloma. Eur J Immunol 1993;23:1289-93. [Crossref] [PubMed]
- Masood A, Christ T, Asif S, et al. Non-secretory multiple myeloma with unusual TFG-ALK fusion showed dramatic response to ALK inhibition. NPJ Genom Med 2021;6:23. [Crossref] [PubMed]
- Papanikolaou X, Zhang Q, Heuck C, et al. Non-Producing Multiple Myeloma (MM) Is a Distinct Subset Of Non-Secretory MM Characterized By High Cyclin D1 Expression and Decreased Progression Free Survival. Blood 2013;122:1911. [Crossref]
- Charliński G, Jurczyszyn A. Non-secretory multiple myeloma: Diagnosis and management. Adv Clin Exp Med 2022;31:95-100. [Crossref] [PubMed]
- Garderet L, D’Souza A, Jacobs P, et al. Response Assessment in Myeloma: Practical Manual on Consistent Reporting in an Era of Dramatic Therapeutic Advances. Biol Blood Marrow Transplant 2017;23:1193-202. [Crossref] [PubMed]
- Dimopoulos MA, Kastritis E, Rosinol L, et al. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 2008;22:1485-93. [Crossref] [PubMed]
- Ludwig H, Pohl G, Osterborg A. Anemia in multiple myeloma. Clin Adv Hematol Oncol 2004;2:233-41. [PubMed]
- Corso A, Mangiacavalli S. Non-Secretory Myeloma: Ready for a new Definition? Mediterr J Hematol Infect Dis 2017;9:e2017053. [Crossref] [PubMed]
- Atrash S, Abdallah AO, Epstein J, et al. Conversion of Non-Secretory to Hyper-Secretory Myeloma: The Antithesis of the Common Pathway of Dedifferentiation with Myeloma Progression: A Case Study. Blood 2012;120:5052. [Crossref]
- Giles HV, Wechalekar A, Pratt G. The potential role of mass spectrometry for the identification and monitoring of patients with plasma cell disorders: Where are we now and which questions remain unanswered? Br J Haematol 2022;198:641-53. [Crossref] [PubMed]
Cite this article as: Lim SM, Ling PC, Loh TP. Woman with cryptic chronic back pain: a case report. J Lab Precis Med 2023;8:32.