
Biological variation estimates for serum N-terminal pro-B-type natriuretic peptide from 8 healthy Turkish female individuals
Introduction
B-type natriuretic peptide (BNP) and its precursor N-terminal pro-BNP (NT-proBNP) are released primarily from cardiac ventricular myocytes in response to increased wall stress and stretch and play a crucial role in the diagnosis, prognosis, and management of various cardiovascular conditions (1,2). NT-proBNP measurements have become integral in the evaluation and monitoring of congestive heart failure (CHF), providing valuable insights into disease severity and response to treatment (3).
The considerable biological variation (BV) observed in BNP/NT-proBNP poses a potential constraint on their practicality for serial measurements in individual patients (4,5). Nevertheless, initial investigations demonstrated that employing BNP/NT-proBNP guidance for heart failure treatment led to a reduction in overall cardiovascular events and a delay in the occurrence of the first event compared to intensive clinically guided treatment (6).
BNP/NT-proBNP values’ ability to aid in risk stratification, guide therapeutic decisions, and predict clinical outcomes has made these measurements an indispensable tool for clinicians. Therefore, it is crucial to understand and measure various sources of variation to accurately interpret changes in serial measurements within an individual. These sources include within-subject BV (CVI), which represents random fluctuations around the individual’s homeostatic set point (HSP), and between-subject BV (CVG), which represents variation among different individuals’ set points (7).
Since laboratory results are typically reported as single values, not considering BV can lead to misinterpretation and potentially unnecessary clinical interventions. Thus, having knowledge of the BV for a particular clinical test allows for proper interpretation of changes between serial measurements, and the statistically significant difference is reported as the reference change value (RCV) (8).
Knowledge of the BV of measurands serves multiple important purposes. It aids in defining analytical performance specifications (APS) (9,10), establishing personalized reference intervals (prRI) for individuals (11,12), evaluating the appropriateness of population-based reference intervals (popRI) through the index of individuality (II), and determining the number of samples required to estimate homeostatic set point (NHSP) within a certain percentage of the true value (13).
Data on BV of NT-proBNP have been published with markedly differing results [reviewed in the study by Wu (14)].
However, to the best of our knowledge, there are limited published studies (4,15-19) that examine the BV of NT-proBNP in healthy individuals, which do not meet the eligibility criteria outlined by the Biological Variation Data Critical Appraisal Checklist (BIVAC) for inclusion in meta-analyses, as established by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) (20).
In our current study, we investigated the BV of NT-proBNP in eight apparently healthy Turkish females, adhering to all the necessary preanalytical requirements outlined by the European Biological Variation Study (EuBIVAS) (21,22) in agreement to the BIVAC and the checklist provided by the working and task groups of BV of EFLM (20,23).
Methods
Study population and protocol
BV estimates for NT-proBNP were obtained from a Turkish population using serum samples collected from apparently healthy individuals, both males and females, enrolled following the methods previously described (24). The health status, inclusion/exclusion criteria, sample collection, processing, and storage protocol were based on the EuBIVAS, previously reported in detail (21,22). Briefly, exclusion criteria included the diagnosis of diabetes mellitus, dyslipidemia, chronic kidney and liver diseases, thalassemia and hemoglobinopathies, carrier status for hepatitis B, C, and human immunodeficiency virus (HIV), and female subjects who were pregnant or breastfeeding. In the first week, all participants underwent a series of laboratory tests to ensure they met the inclusion criteria. The subjects’ use of medications and dietary supplements was recorded on a weekly basis (24).
The sample collection was carried out at Acibadem Labmed Clinical Laboratories and Acibadem Mehmet Ali Aydınlar University in Istanbul, Turkey. The study protocol received approval from the Institutional Ethical Review Board of Acibadem Mehmet Ali Aydınlar University in accordance with the World Medical Association Declaration of Helsinki.
The same phlebotomist collected serum samples on a weekly basis from 24 healthy subjects (12 male and 12 female) over a 10-week period, (February–April 2018), using Vacutainer Serum Separator Tubes (BD Gold, Franklin Lakes, NJ, USA). After centrifugation at 3,000 g, the serum was aliquoted and stored at −80 ℃. The samples were shipped in dry ice to Siemens Healthcare Diagnostics Inc., in Tarrytown, NY, USA, where the measurements were conducted. The samples remained stored at −80 ℃ until the analysis.
Determination of NT-proBNP was performed on the Siemens ADVIA Centaur® XP (Siemens Healthineers, Tarrytown, NY, USA) in December 2022, using the ADVIA Centaur® NT-proBNP (PBNP) assay, and the ADVIA Centaur® NT-proBNP calibrator.
The assay is designed to have a limit of blank (LoB) of less than or equal to the limit of detection (LoD), LoD less than or equal to 20 pg/mL, and limit of quantitation (LoQ) of less than or equal to 35 pg/mL.
Samples from each subject were analyzed in duplicate within a single run, and ADVIA Centaur® NT-proBNP quality control (QC) at two different levels of concentration were used as internal controls of quality and were evaluated in duplicate for each run.
Data analysis
To obtain the BVs and analytical variation coefficient (CVA) estimates with 95% confidence interval (CI), outlier detection, variance homogeneity analyses, and trend analysis followed by CV-ANOVA were conducted following the methodology described in previous EuBIVAS publications (25-27).
Initially, the measurement results lower than LoQ were excluded from the study. Outlier detection was performed using the Dixon-Q test to identify and exclude any outliers between subjects. Subsequently, the homogeneity of within-subject and analytical variability was assessed using the Bartlett and Cochran tests, respectively, on data transformed to coefficient of variation (CV). To determine if individuals were in a steady-state condition, a linear regression analysis between the mean concentrations of duplicate analyses from each blood drawing (representing the pooled mean group samples) and the corresponding blood drawing numbers (1, 2, …, 10) was performed. Subjects were considered to be in a steady state if the 95% CI of the slope included zero.
CVI estimates were derived using the CV-ANOVA Røraas method (28). For estimating CVG, ANOVA was performed on data that were natural log-transformed, and the normality of mean value data distributions was verified using the Kolmogorov-Smirnov test. The 95% CIs for BV estimates were calculated using the Burdick and Graybill method (29).
The following equations were utilized to calculate the desirable APS for analytical imprecision (CVAPS), analytical bias (BAPS), and the II:
The calculation for the asymmetrical RCV and for the NHSP, using the CVA obtained from the duplicate measurements results of subjects’ samples, were performed as previously described (24,25). NHSP were estimated for 15% and 20% deviation from the true HSPs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Acibadem Mehmet Ali Aydınlar University (No. ATADEK - 2023-07/251) and informed consent was obtained from all individual participants.
Results
NT-proBNP results from 11 males out of 12 and three females out of 12 were lower than the LoQ of the assay (<35 pg/mL). The only male with detected results was not included in the analysis. Additionally, one female was identified as an outlier between individuals and was not included in the analysis.
Consequently, the population used to derive the BV of NT-proBNP, consisted of 8 females, median ages of 23.2 years (range, 20–29 years). Physical activity, drug consumption, and smoking and alcohol intake of the study population are summarized in Table 1.
Table 1
| Subjects | Females (n=8) |
|---|---|
| Age, years, median [range] | 23.2 [20–29] |
| BMI, kg/m2, median [range] | 21.6 [17–26] |
| Physical activity, n | |
| No physical activity | 5 |
| >0 to <3 h/week | 1 |
| ≥3 h/week | 2 |
| Smoking habits, n | |
| 0 cigarette/day | 6 |
| >0 to <10 cigarettes/day | 2 |
| 10–20 cigarettes/day | 0 |
| Drug consumption, n | |
| No drug | 8 |
| Type of drug | – |
| Alcohol intake†, n | |
| 0 U/day | 6 |
| >0 to <2 U/day | 2 |
| ≥2 U/day | – |
†, one alcohol unit (U) correspond to 10 mL, equivalent to 8 grams, of pure alcohol (https://www.drinkaware.co.uk/alcohol-facts/alcoholic-drinks-units/what-is-an-alcohol-unit/). BMI, body max index.
To fulfil criteria for variance homogeneity of the data, 7.1% of results were excluded (Table 2). In total, 104 analytical results were included in the analysis. NT-proBNP data were normally distributed and no trends were identified by regression analysis. The CVI and CVG estimates were 23.7% (95% CI: 19.0–30.4%) and 8.22% (95% CI: 0–24.7%) respectively (Table 3).
Table 2
| Subjects | Females |
|---|---|
| Homogeneity (Bartlett and Cochran’s tests), n | |
| Replicate (analytical homogeneity) | 0 |
| Samples (within homogeneity) | 4 |
| Subjects (within homogeneity) | 0 |
| Reed and Dixon, n | |
| Subjects (between) | 1 |
| Numbers of results used to estimate CVI data, n | |
| Results | 104 |
| Subjects | 8 |
| % of outliers excluded to estimates CVI data | 7.1 |
CVI, within-subject biological variation.
Table 3
| Subjects | Females |
|---|---|
| Number of individuals | 8 |
| Total number of results | 104 |
| Mean number of samples/individual | 6.63 |
| Mean number of replicates/sample | 1.93 |
| Mean value, pg/mL (95% CI) | 54.1 (50.7–57.6) |
| CVA %1 (95% CI) | 11.2 (9.4–13.9) |
| CVI % (95% CI) | 23.7 (19.0–30.4) |
| CVG % (95% CI) | 8.22 (0–24.7) |
1, CVA estimates were based on CV-ANOVA of duplicate analysis of female study samples. CVI, within-subject biological variation; CVG, between-subject biological variation; NT-proBNP, N-terminal pro-B-type natriuretic peptide; CI, confidence interval; CVA, analytical variation coefficient.
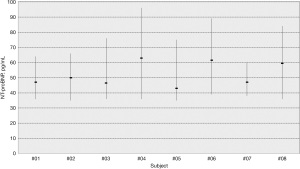
The APS for imprecision and bias, RCV, II and NHSP were calculated (Table 4). In Figure 1, median values and range of NT-proBNP concentrations for each individual are shown.
Table 4
| Variable | Data |
|---|---|
| CVAPS %a | |
| Minimum | 17.8 |
| Desirable | 11.8 |
| Optimum | 5.9 |
| BAPS %b | |
| Minimum | 17.8 |
| Desirable | 6.3 |
| Optimum | 5.9 |
| RCV %c (decrease; increase) | −45.2; 82.4 |
| IId | 2.7 |
| NHSPe, n | |
| 15% | 12 |
| 20% | 7 |
a, CVAPS = 0.5 CVI (desirable level). The factor for optimum and minimum performance specifications are arbitrarily set to 0.25 and 0.75 respectively. b, BAPS = 0.25 (CVI2 + CVG2)0.5 (desirable level). The factor for optimum and minimum performance specifications are 0.125 and 0.375, respectively. c, RCV were calculated as described in the text delivering asymmetric values for rise and fall at the probability level of 95% for significant unidirectional change, applying CVA estimates based on duplicate measurement of all study samples. d, II = CVI/CVG. e, NHSP [Z*(CVA2 + CVI2)1/2/D]2 where D is the allowed percentage deviation from the true homeostatic set point, and Z is 1.96 (for a P value <0.05). NHSPs associated with 15%, and 20% deviations from the true homeostatic set points are calculated. APS, analytical performance specification; CVAPS, analytical performance specification for imprecision; BAPS, analytical performance specification for bias; RCV, reference change values; II, index of individuality; NHSP, number of samples required to estimate the homeostatic set point; NT-proBNP, N-terminal pro-B-type natriuretic peptide; BV, biological variation; CVI, within-subject biological variation; CVG, between-subject biological variation; CVA, analytical variation coefficient.
Discussion
This study exhibits a rigorous control over both the preanalytical and analytical phases. The study design and data processing were meticulously planned and implemented in accordance with the recommendations set forth by the EFLM (20,23) and adhered strictly to the EuBIVAS protocol (21,22).
The clinical relevance of NT-proBNP in the management of heart failure is well-acknowledged, with international guidelines emphasizing its significance in the diagnostic, prognostic, and therapeutic contexts. Specifically, it has become an indispensable tool for the evaluation of adult patients, primarily those over the age of 55 years, who suffer from heart failure (1).
In the present study, we determined the BV of serum NT-proBNP in 8 healthy Turkish female individuals with a median age of 23.2 years (range, 20–29 years). It is crucial to recognize the potential limitations this might pose. While the demographic investigated provided valuable insights, it is not entirely representative of the typical heart failure patient, often being older adults of both genders. This discrepancy could influence the generalizability of our findings to the broader patient population. However, it is noteworthy that understanding the BV in a younger, healthy demographic can provide a foundation upon which future studies can build.
Results from male samples were below the LoQ of the assay (<35 pg/mL) for 11 out of the 12 samples and hence BV estimates could not be derived. This is in line with recent reports indicating sex-based differences in healthy populations (30). The lower circulating androgens and the potentiating effect of exogenous female hormone therapy contribute to the higher circulating NT-proBNP concentrations in females (31).
Specifically, females tend to exhibit higher NT-proBNP concentrations compared to males from late adolescence until middle age and this suggests that age- and sex-specific intervals may be necessary to more accurately assess the associated risk levels.
Interestingly, the previous studies did not provide differentiated BV estimates for males and females, despite reporting differences in concentration between the two sexes (17). Consequently, the CVG estimates were incorrectly obtained by combining two heterogeneous groups, leading to a misleading overestimation of the CVG value and consequently a misleading value of the BAPS and II.
For the measurands with high individuality, where the CVI is small if compared to the CVG (defined as II = CVI/CVG), for a correct interpretation of serial results, the use of the reference intervals (RIs) should be replaced by the RCV. In such situations, the individual is the best point of reference for assessing change, armed with the knowledge of the CVI of the measurand. The II has been in fact proposed as a discriminator for the usefulness of RCV values. II values lower than 0.6 indicate marked individuality, while RIs are suitable for the assessment of measurands with an II >1.4 (7).
The II observed in this study, was found to be 2.7. This finding implies a diminished degree of individual variability in NT-proBNP.
Therefore, employing a RI for females based on the population, appears suitable for interpreting successive NT-ProBNP measurements. However, the traditional perspective on the use of the RCV should be reassessed considering emerging evidence that underscores the significance of personalized RI. Carobene et al. using EuBIVAS data, demonstrated that when calculating CVI in the single individual (referred to as CVP), the resulting values exhibited a surprisingly broad range that was independent of the measurands concentration (12). This indicates that despite a reduced level of individuality within the population, there can still be advantages in utilizing a prRI based on the individual’s own data. To simplify the concept, a glance at Figure 1 depicting the data of individual subjects is sufficient to observe that the intra-individual variability of subject 7 (CVP =17.4%) is considerably lower compared to subject 4 (CVP =27.7%).
Utilizing the CVA and CVI estimates derived from the entire population described in this study, the generalized asymmetrical RCVs were determined to be −45.2% to 82.4% (decrease to increase), as shown in Table 4. Nevertheless by applying CVA estimates based on duplicate measurements of all study samples and utilizing CVPs instead of CVI, the RCVs obtained were −37.9% to 61.1% and −49.4% to 97.7% for subject 7 and subject 4, respectively.
It means that to positively impact the patient’s report, it is essential to consider all relevant variables that characterize our patients (11). To note that this evaluation requires significant effort to be effective. In fact, key unanswered questions, including determining the reliability of homeostatic setting points and CVP values based on the number of patient results, addressing uncertainty related to analytical components, updating prediction intervals with new results, and incorporating physiological drift due to seasonal and age-related factors, still remains (32). Addressing these questions requires dedicated effort and ongoing studies to provide comprehensive solutions. It is through these continued efforts that we can enhance the patient’s report and improve clinical practice.
The CVA estimate obtained in our study is below the desired. This would suggest that the analytical system employed in our study likely meets these requirements. However, the measurement uncertainty does not follow a linear pattern between the LoQ and the upper measurement limit. It increases near both the LoQ and the upper measurement limit. Therefore, if the measurement result of the analyte falls near the LoQ or the upper measurement limits, the standard deviation (SD) and, consequently, the total variation will be higher. Additionally, if the measurement result is below the LoQ, it cannot be accepted as reliable data, and these results should not be incorporated into further calculations.
These issues highlight the limitations of the existing methods. In cases where such limitations arise, the method should be refined to accurately measure the lower levels of the analyte, which correspond to the normal levels found in healthy individuals.
Moreover, it is important to consider the strict pre-analytical protocol employed in this study according to the EuBIVAS (21,22), and the fact that the CVA estimates are based on duplicate analysis of all samples. In routine settings with long-term analysis, it is possible for the CVA estimates to surpass the APS.
In this study, another noteworthy discovery was the identification of NHSPs for NT-proBNP, with values of seven and 12 accompanied by an approximate deviation of 20% and 15% respectively (Table 4). This suggests that when the NT-proBNP concentration is close to the action limit, clinical decision-making might require multiple measurements to ensure accurate interpretation.
Until now, to the best of our knowledge, six papers (4,15-19) have been published that examine the BV of NT-proBNP in healthy individuals. Two of which (18,19) reported BV data diurnal variation, therefore, according to the BIVAC they are not eligible to be included in the meta-analysis.
In the remaining four published papers, CVI estimates range from 10% (16) up to 58% (15). Three of these papers, published prior to the EFLM recommendations, reported BV estimates without CIS measures: this presents a challenge when attempting to make a direct comparison between these historical data and the new data presented in this study. Furthermore, these studies would have been categorized as BIVAC grade C. When assessed using the BIVAC (20) quality evaluation, certain BIVAC quality items (QIs), specifically QI 7 (steady state) and QI 10 (variance homogeneity), were not satisfied. It is likely that publications lacking or inadequately addressing essential details related to BIVAC QIs may result in less reliable CVI estimates (33).
Limitation
It is essential to recognize certain limitations inherent to our study, which should be taken into account in the planning of future research projects. This study exhibits a limitation related to its small sample size while maintaining the rigorous control over both the preanalytical and analytical phases. We were in fact limited by the ability to measure below the LoQ and hence were not able to get results for males because, apart from a single exception, all the NT-proBNP males’ results were lower than the LoQ.
Another limitation of this study is the age demographic of the sampled population. While this provides valuable insights into the BV of NT-proBNP in this specific age group, it may not be entirely representative of the broader population, especially those most clinically relevant to heart failure.
Moreover, the analyses were performed using only one manufacturer’s reagents. However, estimates of BV that describe the natural fluctuations of the measured quantity should not be influenced by the specific reagent used, as demonstrated in previous EuBIVAS studies (34,35).
Nevertheless, our findings provide a foundational understanding of NT-proBNP BV in the studied group, which could serve as a stepping stone for subsequent research in more clinically relevant populations. It would be beneficial for future studies to expand on this work by including a more diverse age group, with a particular focus on the heart failure demographic.
Conclusions
In conclusion, the fully BIVAC-compliant BV data on NT-proBNP represent an update of the presently available data, some of them obtained more than 20 years ago. In our study, CVI estimate was homogeneously distributed and lower than previously published estimates, which means that more stringent CVAPS were derived.
Acknowledgments
This study was supported by Siemens Healthineers.
Funding: None.
Footnote
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-59/coif) and report that this study was supported by Siemens Healthineers. C.D. is an employee of Siemens Healthineers. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Acibadem Mehmet Ali Aydınlar University (No. ATADEK - 2023-07/251) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- deFilippi CR, Christenson RH, Gottdiener JS, et al. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol 2010;55:441-50. [Crossref] [PubMed]
- Writing Committee Members. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [PubMed]
- Klersy C, d'Eril GV, Barassi A, et al. Advantages of the lognormal approach to determining reference change values for N-terminal propeptide B-type natriuretic peptide. Clin Chim Acta 2012;413:544-7. [Crossref] [PubMed]
- Miller WL, Hartman KA, Grill DE, et al. Only large reductions in concentrations of natriuretic peptides (BNP and NT-proBNP) are associated with improved outcome in ambulatory patients with chronic heart failure. Clin Chem 2009;55:78-84. [Crossref] [PubMed]
- Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000;355:1126-30. [Crossref] [PubMed]
- Fraser CG. Biological Variation: From Principles to Practice. Washington, DC: Amer. Assoc. for Clinical Chemistry; 2001.
- Fraser CG. Reference change values: the way forward in monitoring. Ann Clin Biochem 2009;46:264-5. [Crossref] [PubMed]
- Carobene A, Franzini C, Ceriotti F. Comparison of the results from two different External Quality Assessment Schemes supports the utility of robust quality specifications. Clin Chem Lab Med 2011;49:1143-9. [Crossref] [PubMed]
- Haeckel R, Wosniok W, Kratochvila J, et al. A pragmatic proposal for permissible limits in external quality assessment schemes with a compromise between biological variation and the state of the art. Clin Chem Lab Med 2012;50:833-9. [Crossref] [PubMed]
- Coşkun A, Sandberg S, Unsal I, et al. Personalized Reference Intervals in Laboratory Medicine: A New Model Based on Within-Subject Biological Variation. Clin Chem 2021;67:374-84. [Crossref] [PubMed]
- Carobene A, Banfi G, Locatelli M, et al. Within-person biological variation estimates from the European Biological Variation Study (EuBIVAS) for serum potassium and creatinine used to obtain personalized reference intervals. Clin Chim Acta 2021;523:205-7. [Crossref] [PubMed]
- Fraser CG, Browning MC. The “index of fiduciality” proposed for use in evaluation and comparison of methods. Clinical Chemistry 1988;34:1356-7. [Crossref] [PubMed]
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J 2006;152:828-34. [Crossref] [PubMed]
- Wu AH, Smith A, Wieczorek S, et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 2003;92:628-31. [Crossref] [PubMed]
- Melzi d'Eril G, Tagnochetti T, Nauti A, et al. Biological variation of N-terminal pro-brain natriuretic peptide in healthy individuals. Clin Chem 2003;49:1554-5. [Crossref] [PubMed]
- Meijers WC, van der Velde AR, Muller Kobold AC, et al. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail 2017;19:357-65. [Crossref] [PubMed]
- Goetze JP, Jørgensen HL, Sennels HP, et al. Diurnal plasma concentrations of natriuretic propeptides in healthy young males. Clin Chem 2012;58:789-92. [Crossref] [PubMed]
- Sothern RB, Vesely DL, Kanabrocki EL, et al. Blood pressure and atrial natriuretic peptides correlate throughout the day. Am Heart J 1995;129:907-16. [Crossref] [PubMed]
- Aarsand AK, Røraas T, Fernandez-Calle P, et al. The Biological Variation Data Critical Appraisal Checklist: A Standard for Evaluating Studies on Biological Variation. Clin Chem 2018;64:501-14. [Crossref] [PubMed]
- Carobene A, Aarsand AK, Bartlett WA, et al. The European Biological Variation Study (EuBIVAS): a summary report. Clin Chem Lab Med 2022;60:505-17. [Crossref] [PubMed]
- Carobene A, Strollo M, Jonker N, et al. Sample collections from healthy volunteers for biological variation estimates' update: a new project undertaken by the Working Group on Biological Variation established by the European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med 2016;54:1599-608. [Crossref] [PubMed]
- Bartlett WA, Braga F, Carobene A, et al. A checklist for critical appraisal of studies of biological variation. Clin Chem Lab Med 2015;53:879-85. [Crossref] [PubMed]
- Coşkun A, Carobene A, Aarsand AK, et al. Within- and between-subject biological variation data for serum zinc, copper and selenium obtained from 68 apparently healthy Turkish subjects. Clin Chem Lab Med 2022;60:533-42. [Crossref] [PubMed]
- Cavalier E, Lukas P, Bottani M, et al. European Biological Variation Study (EuBIVAS): within- and between-subject biological variation estimates of β-isomerized C-terminal telopeptide of type I collagen (β-CTX), N-terminal propeptide of type I collagen (PINP), osteocalcin, intact fibroblast growth factor 23 and uncarboxylated-unphosphorylated matrix-Gla protein-a cooperation between the EFLM Working Group on Biological Variation and the International Osteoporosis Foundation-International Federation of Clinical Chemistry Committee on Bone Metabolism. Osteoporos Int 2020;31:1461-70. [Crossref] [PubMed]
- Bottani M, Aarsand AK, Banfi G, et al. European Biological Variation Study (EuBIVAS): within- and between-subject biological variation estimates for serum thyroid biomarkers based on weekly samplings from 91 healthy participants. Clin Chem Lab Med 2022;60:523-32. [Crossref] [PubMed]
- Clouet-Foraison N, Marcovina SM, Guerra E, et al. Analytical Performance Specifications for Lipoprotein(a), Apolipoprotein B-100, and Apolipoprotein A-I Using the Biological Variation Model in the EuBIVAS Population. Clin Chem 2020;66:727-36. [Crossref] [PubMed]
- Røraas T, Støve B, Petersen PH, et al. Biological Variation: The Effect of Different Distributions on Estimated Within-Person Variation and Reference Change Values. Clin Chem 2016;62:725-36. [Crossref] [PubMed]
- Burdick RK, Graybill FA. Confidence Intervals on Variance Components. 1st ed. New York: Marcel Dekker, Inc.; 1992.
- Mu S, Echouffo-Tcheugui JB, Ndumele CE, et al. NT-proBNP Reference Intervals in Healthy U.S. Children, Adolescents, and Adults. J Appl Lab Med 2023;8:700-12. [Crossref] [PubMed]
- Lam CS, Cheng S, Choong K, et al. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol 2011;58:618-26. [Crossref] [PubMed]
- Carobene A, Banfi G, Locatelli M, et al. Personalized reference intervals: From the statistical significance to the clinical usefulness. Clin Chim Acta 2022;524:203-4. [Crossref] [PubMed]
- Carobene A, Guerra E, Locatelli M, et al. Providing Correct Estimates of Biological Variation-Not an Easy Task. The Example of S100-β Protein and Neuron-Specific Enolase. Clin Chem 2018;64:1537-9. [Crossref] [PubMed]
- Ceriotti F, Díaz-Garzón Marco J, Fernández-Calle P, et al. The European Biological Variation Study (EuBIVAS): weekly biological variation of cardiac troponin I estimated by the use of two different high-sensitivity cardiac troponin I assays. Clin Chem Lab Med 2020;58:1741-7. [Crossref] [PubMed]
- Carobene A, Marino I, Coşkun A, et al. The EuBIVAS Project: Within- and Between-Subject Biological Variation Data for Serum Creatinine Using Enzymatic and Alkaline Picrate Methods and Implications for Monitoring. Clin Chem 2017;63:1527-36. [Crossref] [PubMed]
Cite this article as: Carobene A, Abou-Diwan C, Locatelli M, Serteser M, Coskun A, Unsal I. Biological variation estimates for serum N-terminal pro-B-type natriuretic peptide from 8 healthy Turkish female individuals. J Lab Precis Med 2024;9:3.


