
Green neutrophilic inclusions in patient with drug intoxication: a case report
Highlight box
Key findings
• Blue-green inclusion, acute liver failure, hematology laboratory.
What is known and what is new?
• Blue-green inclusions are an unusual cytoplasmic finding in neutrophils in peripheral blood smear. They have been related to acute liver failure and it is important to notify them due to the poor prognosis of the patient.
• We provide new graphic documentation of these rare inclusions related to a case of multidrug intoxication.
What is the implication, and what should change now?
• It is necessary that professional of laboratories know the importance of informing the doctor of the findings of these inclusions and their relation with impending death.
Introduction
Intracytoplasmic blue-green inclusions in leukocytes are a rare finding usually associated with high mortality. This type of inclusion bodies has also been called “green crystals of death” because their prognostic value showed a mortality rate as high as 65%, with 92% dying within 72 hours after admission (1). These inclusions appear mainly on neutrophils, although with a lower percentage they can also be seen in monocytes. Here, we presented intracytoplasmic blue-green inclusions a patient with drug-induced acute liver failure. We present this case in accordance with the CARE reporting checklist (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-30/rc).
Case presentation
A 54-year-old woman was admitted to the emergency department with clinical suspicion of multi-drug and alcoholic intoxication. She was found lying on her home’s floor next to rum and vodka bottles and empty blisters of several drugs, including acetaminophen, diclofenac, amoxicillin, lormetazepam, dexketoprofen and clorazepate dipotassium. Physical examination showed tachypnea, poor perfusion and dehydration signs. No further data of interest in her personal medical history except a mild anxiety-depressive mixed disorder.
On admission, blood analysis revealed severe metabolic acidosis (pH <7.00, lactic acid >17 mmol/L), hyperglycemia (glucose, 512 mg/dL), liver damage [alanine transaminase (ALT), 3,527 U/L] and severe coagulopathy [international normalized ratio (INR), 3.3; fibrinogen, 185 mg/dL] (Table 1, day 1).
Table 1
| Variables | Hospitalization day | Reference values | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Glucose (mg/dL) | 512 | 428 | 143 | 67–110 |
| AST (U/L) | – | 16,602 | 29,452 | 5–47 |
| ALT (U/L) | 3,527 | 5,464 | 7,046 | 5–47 |
| GGT (U/L) | – | 268 | 379 | 5–40 |
| Alkaline phosphatase (U/L) | – | 104 | 219 | 30–106 |
| Bilirrubin (mg/dL) | 2.2 | 3.7 | 3.4 | 0.2–1.2 |
| Amylase (U/L) | 1,296 | 2,572 | 2,478 | 22–108 |
| LDH (U/L) | 8,191 | 25,801 | 19,755 | 26–245 |
| Lactate (mmol/L) | >17 | >17 | >17 | 0.55–1.98 |
| pH | <7.00 | 7.24 | 7.17 | 7.35–7.45 |
| Creatinine (mg/dL) | 2.07 | 1.78 | 1.28 | 0.55–1.10 |
| Hemoglobin (g/dL) | 9.1 | 9.8 | 8.4 | 12–16 |
| Neutrophils (×103/µL) | 5.62 | 5.53 | 5.44 | 2–5 |
| Lymphocytes (×103/µL) | 1.11 | 0.97 | 0.65 | 1.3–2.9 |
| Monocytes (×103/µL) | 0.17 | 0.16 | 0.24 | 0–0.8 |
| Platelets (×103/µL) | 41 | 17 | 18 | 135–450 |
| INR | 3.3 | 6.5 | >10 | 0.8–1.2 |
| APTT (s) | 74 | 87 | 119 | 22–32 |
| Fibrinogen (mg/dL) | 185 | 46 | <40 | 180–400 |
| Blue-green inclusions/200 neutrophils | – | 2 | 2 | – |
AST, aspartate transaminase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase; INR, international normalized ratio; APTT, activated partial thromboplastin time.
She was transferred to the intensive care unit due to hemodynamic shock. She also presented Acute Kidney Injury Network (AKIN) (full term stage) III renal failure, pancreatic insufficiency and fulminant liver failure. Unsuccessful attempts to reverse the critical situation of the patient were made with the administration of vitamin K, bicarbonate, insulin, platelet pool, fibrinogen and increasing doses of vasoactives and inotropes.
Despite intensive organ support treatment, progressive deterioration of the liver function occurred with evidence of incoagulability, catastrophic hemodynamic instability, and generalized perfusion deficit. Due to her terminal situation, the patient was not suitable for liver transplantation, and finally, she died 72 hours after admission into the intensive care unit.
On the second day of admission, peripheral blood smear examination revealed blue-green inclusions in 1% of the neutrophils (Figure 1). These intracellular crystals have been previously described in the literature as indicators of poor prognosis (2).
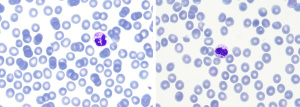
Given the patient’s terminal situation, with known liver failure and the impossibility of transplantation, we considered not to report them, since the finding did not provide further information to the clinician. In agreement with the published bibliography, death occurred in a short period after observation of the crystals (3). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient, because her deceased, or the relatives after all possible attempts were made.
Discussion
Blue-green inclusion bodies found within the cytoplasm of neutrophils are a rare clinical finding. Their exact origin and their composition are yet to be determined, but current hypotheses suggest that the inclusions likely originate from a lipofuscin-like substance produced by the injured liver and taken up by neutrophils and macrophages (4).
Peripheral blood smear examination is the only procedure that allows inclusion detection, as they cannot be detected by hematology analyzers. Previous studies indicate that it is highly important to report the presence of these inclusions in unknown liver disease cases, given its relation with impending death (5). For this reason, we believe that by providing more graphic documentation of these inclusions, we can help other professionals to identify them.
Conclusions
Our case supports the current published literature and serves as a reminder of the importance of blue-green inclusions on peripheral blood smear. These crystals, in the context of acute liver failure and lactic acidosis, could be predictors of mortality in critically ill patients (3). Therefore, information provided by the laboratory is essential for the patient’s prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-30/rc
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-30/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-30/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient, because her deceased, or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vicente-Steijn R, Tomé A, Maduell F, et al. Green inclusions in neutrophils: A critical finding that must be reported. Int J Lab Hematol 2020;42:e101-4. [Crossref] [PubMed]
- Harris VN, Malysz J, Smith MD. Green neutrophilic inclusions in liver disease. J Clin Pathol 2009;62:853-4. [Crossref] [PubMed]
- Hodgson TO, Ruskova A, Shugg CJ, et al. Green neutrophil and monocyte inclusions - time to acknowledge and report. Br J Haematol 2015;170:229-35. [Crossref] [PubMed]
- Soos MP, Heideman C, Shumway C, et al. Blue-green neutrophilic inclusion bodies in the critically ill patient. Clin Case Rep 2019;7:1249-52. [Crossref] [PubMed]
- Merino A, Molina A, Rodríguez-García M, et al. Detection and significance of green inclusions in peripheral blood neutrophils and monocytes. Int J Lab Hematol 2021;43:e92-4. [Crossref] [PubMed]
Cite this article as: Rubio I, Martínez de la Puente E, Bóveda O, Jiménez I, Peña I. Green neutrophilic inclusions in patient with drug intoxication: a case report. J Lab Precis Med 2024;9:9.


