Modern hematology analyzers: beyond the simple blood cells count (with focus on the red blood cells)
Introduction
The modern hematological analyzers are easy to use and can process several blood samples in a short period of time (e.g., 120 samples/h) (1-3). The latest generation of hematological analyzers can also generate accurate results. However, abnormal spurious results can be observed for the leukocyte count, platelets count, nucleated red cells count, haemoglobin, red cell indices and reticulocytes (4,5). Usually, these analyzers are used for carrying out the cell count and the differential leukocyte analysis. However, they can also provide additional information relevant to clinicians. A better knowledge of the potential of these instruments and their limits is therefore crucial. In this paper we present some examples of the potential diagnostic information that the hematological analyzer can provide.
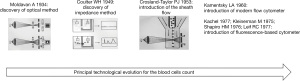
Hematological analyzers: history of an evolving technology
Cell analysis of hematological analyzers relies on two principles: (I) electrical resistance or impedance and (II) optical analysis. The principle of impedance was formally first used by Coulter in 1949 and has enabled cytometers to count and measure the volume of analyzed cells (6).
This method was further perfectionated thanks to the following improvements: hydrodynamic focusing, correction for coincidence, recognition and elimination of abnormal signals, use of lysing agents selective for specific leukocyte populations. In 1968, the production of the Coulter Model-S© with sampling valve and automatic correction for coincidence, revolutionized the activity of hematological laboratories (7,8).
A similar method, based on capacitance- rather than resistance-measurements, was developed in the 1950s in Japan and became the basis for the first analyzers commercially produced by TOA (now Sysmex, Kobe, Japan) (9).
A further extension of Coulter’s principle consisted of the contemporary use of low and high frequency current to examine different cell characteristics: the size in the first case and the composition and internal structure in the latter. These bi-parametric measurements were commercially applied in 1986 first with the volume, conductivity and scatter (VCS) transducers produced by Coulter and in 1988 with the NE series of analyzers produced by Sysmex (9-13).
In flow cytometry the optical method was developed before the impedance method. The first application by Moldavan dates to 1934 (14).
In 1953, the use of dark field analysis and the introduction of sheath flow by Crosland-Taylor were two fundamental steps for obtaining relatively accurate cell counts (15). Following these developments, the first commercial instruments based on optical method to perform the blood cell counts were finally produced.
The amplitude of scattered light collected at narrow angle (forward scatter) was found to correlate to the cell volume, while the refraction index at greater angles was associated with the cellular structural complexity.
In early ’60, Kamentsky et al. built the “Rapid Cell Spectrophotometer”, the first flow cytometer capable to evaluate a cell volume measurement based on scattering and absorbance. In 1974, the combined use of these two methods was commercially applied by Technicon (now Siemens, Erlangen, Germany) in the Hemalog-D© instrument, which was able to perform the complete differential leukocyte count (16).
The red blood cell (RBC) volume measurement using optical method was influenced both by the erythrocyte biconcave discoidal shape and the different refraction index of each cell [depending on hemoglobin (Hb) concentration] (17,18).
The last two decades have been characterized by the application of fluorescence measurements using dyes for specific cell components (i.e., the nucleic acids), or, less frequently, dyes bound to monoclonal antibodies. Currently, the fluorescence measurements are only qualitative or semiquantitative and are utilized by some manufacturers (Abbott, Wiesbaden, Germany; ABX-Horiba, Kyoto, Japan; Sysmex) to obtain the reticulocyte and erythroblast counts or in some cases the differential leukocytes or platelet counts (19-22).
The historical chronology of the principal technological evolution of haematological instrumentation is shown in Figure 1.
Red cell diseases and diagnosis
Erythrocytes abnormalities and defects are frequently found in clinical laboratory medicine. Several authors have attempted to simplify diagnosis by using information offered by hematological analyzers. Already in 1989, Pati et al. reported the advantages of the Technicon H1-analyzerTM (now Siemens) for the diagnosis of hereditary spherocytosis (HS), using the means of mean corpuscular hemoglobin concentration (MCHC) and red blood cell distribution width (RDW) measured by this instrument. Spherocytes were recognizable in the H1 histograms of cell size and Hb concentration as distinctive microcytic and hyperchromic tails (23).
More recently Sugandha et al. reported the utility of RBCs data (percentage of hyperchromic cells, hyperchromic cell flagging, and RBC cytogram pattern) generated by the Advia-120TM hematology analyzer (Siemens) as a potential screening tool for HS (24). Furthermore, Rooney et al. reported that the measurement of hyperchromic erythrocytes is highly sensitive and specific for detecting HS in children using the hematology analyzer Cell-Dyn Sapphire (Abbott) (25).
In addition, Nivaggioni et al. recently even suggested a “decision tree” algorithm based on erythrocytes and reticulocytes parameters obtained by Sysmex XN-series hematology analyzers. This was able to facilitate the diagnosis between HS and iron deficiency anemia (IDA) (26). Nivaggioni et al. created a flow chart for the classification of hereditary RBCs diseases, with a sensibility and specificity for RBC diseases detection of 95.2% and 99.9% respectively (26). The following parameters were used for this: (I) the MCHC, (II) percentage of microcytes, (III) RDW, (IV) percentage of nucleated RBCs and (V) immature reticulocytes fraction.
The same authors proposed afterwards a complementary decision three for the detection of Southern Asian Ovalocytosis (27). Mullier et al. proposed an algorithm for the identification of HS. This tool uses RBCs and reticulocytes parameters provided by the automated hematological analyzers such as the reticulocytes to immature reticulocytes fraction ratio, the percentage of micro-reticulocytes, the ratio between micro-reticulocytes and reticulocytes with low Hb levels (28). It is important to stress that this algorithm was included in the 2015 International Council for Standarization in Haematology Guidelines for the laboratory diagnosis of erythrocytes cell membrane disorders (29). Several other authors validated the algorithm proposed by Mullier with adjusted cut-off according to patient cohort type (30-33).
Urrechaga et al. suggested to use automated measurement of RBC subpopulations to screen efficiently between patients with microcytosis from those with β-thalassemia (34). According to these authors, the use of formula based on the percentage of hypocromic RBC, the percentage of microcytic RBC and RDW can efficiently screen subjects with microcytosis who require further hematological studies to confirm β-thalassemia (sensitivity 100%, specificity 92.6%) (34). Few years before, the same author, reported on the possibility of using an index obtained with the Advia-2120TM (Siemens) (% microcytic/% hypochromic ratio index) to differentiate between microcytic anemia and beta-thalassemia with an excellent rate of success (35). In another study the author evaluated the clinical utility of the “low haemoglobin density”, a new parameter provided by Beckman-Coulter LH750TM (Fullerton, CA, USA) analyzer for the diagnosis of iron deficiency. The conclusion of this study was that this new parameter can determine iron deficit with a specificity and sensibility of 93.3% and 95.2% respectively (36). The parameter low haemoglobin density in addition to some new red cell parameters (RBC size factor, microcytic anaemia factor, standard deviation of conductivity of the non-reticulocyte population and unghosted cell) provided by Beckman Coulter DxH800TM analyzer has been evaluated by Ng et al. The conclusion of these authors was that the new RBC parameters on Beckman Coulter DxH800© provide valid information in distinguishing between IDA from thalassemia trait (37).
In 2012, another algorithm derived from extendend RBC parameters provided by the Sysmex XE5000TM analyzer such as percentage of hyprochromic and microcytic RBCs and Hb content of reticulocytes was proposed as laboratory anemia screening devices (38). Table 1 summarizes some of the main works performed by the various authors to facilitate the diagnosis of red cell abnormalities and diseases.
Table 1
| Authors, Year | Main purpose | Main outcomes |
|---|---|---|
| Mullier et al., 2011 (28) | To develop easy to use diagnostic tool for screening of hereditary spherocytosis based on routinely acquired haematological parameters | The performance of the described HS diagnostic tool show a sensitivity, specificity, positive predictive value and negative predictive value respectively of 100%, 99.3%, 75% and 100% |
| Urrechaga et al., 2011 (34) | To prospectively evaluate the discriminant efficiency of new indices, calculated from RBC extended parameters reported by the Sysmex XE 5000 analyzer, in the differential diagnosis of microcytic anemia and β thalassemia screening | The proposed formula can be used to efficiently screen subjects with microcytosis for further hematological studies to confirm β-thalassemia (sensitivity 100%, specificity 92.6%) |
| Nivaggioni et al., 2020 (26) | To ameliorate the diagnosis between subjects with hereditary RBCs abnormality and subjects with IDA | The elaborated flow chart may be helpful to classify hereditary RBCs diseases, with a sensibility and specificity for RBC diseases detection of 95.2% and 99.9% respectively |
| Roccaforte et al., 2020 (39) | To evaluated the diagnostic performance of the new Iron-Def flag in patients with IDA | The “Iron-Def” parameter can be used as a rapid and inexpensive parameter for initial screening for IDA although further confirmatory investigations are needed |
| Bakri et al., 2020 (40) | To assessed the Ret-He as a screening tool for the diagnosis of IDA in adults | The Ret-He showed a sensitivity of 89.32% and a specificity of 100% when compared to transferrin saturation. The authors concluded that Ret-He together with a complete blood count may serve as an alternative to the serum iron parameters for screening of IDA in adults |
HS, hereditary spherocytosis; RBC, red blood cell; IDA, iron deficiency anemia; Iron-Def, iron deficiency; Ret-He, reticulocyte-hemoglobin equivalent.
Another example is the semi-quantitative parameter, defined “Iron Deficiency?” (Iron-Def), automatically provided by the XN-seriesTM analyzers. The parameter “Iron-Def” has values ranging between 50 and 110 and is triggered when MCHC and mean corpuscular volume (MCV) values are decreased and simultaneously the RDW-coefficient of variation is increased. The definitive underlying conditions of the flags are defined as “confidential” by Sysmex. Recently, Roccaforte et al. investigated the diagnostic performance of the new Sysmex XNTM (Iron-Def) parameter for the diagnosis of patients with IDA. The accuracy of Iron-Def in the identification of patients with a percentage of transferrin saturation <15% (n=104) was 84%, with a sensitivity of 95.2% and specificity of 53.8%. A sub-analysis of 71 patients with ferritin <20 ng/dL yielded an even better diagnostic performance (86%, with a sensitivity of 93.5% and specificity of 62.0%) and the conclusion of the authors was that “Iron-Def” may be used as the inexpensive and rapid parameter for initial screening for IDA, although further confirmatory investigations are needed (39).
The latest generation of hematology analyzers provides some reticulocyte indices analogous to the equivalent RBC indices. Among these, the most promising from a clinical point of view are the Hb content of the reticulocyte and the mean reticulocyte volume (41). Several new reticulocyte parameters or indices are provided by different hematological instruments, the reticulocyte hemoglobin content (CHr) (ADVIA, Siemens), mean reticulocyte hemoglobin content (MCHr) (CELL-DYN Sapphire, Abbott), reticulocyte hemoglobin equivalent (RHE) (BC-6800, Mindray, Shenzhen, China) and reticulocyte Hb content calculated RHCc (Petra Nexus DX, ABX-Horiba), whose clinical and diagnostic meaning in the evaluation of IDA can be considered practically equivalent (40,42-44).
Cold agglutinin and analyzers
The presence of cold agglutinins is often a random laboratory finding associated with difficulties in performing complete blood count (CBC) with a spurious increase in the MCHC and decrease in the RBCs. Several methods have been used to inhibit the interference of cold agglutinins with the analyzer. The most widely used consist in incubating the blood sample in a hot water bath at 37 ℃ for 2 hours. Recently, our group evaluated the possibility to use the reticulocyte channel on the XN-analyzer, heated to 41 ℃, to analyze samples with cold agglutinins. Furthermore, CBCs obtained in reticulocyte channel were compared to those obtained with the traditional impedance method for the RBCs count on the XN analyzer after preheating samples to 37 ℃ for 2 hours. The conclusion of the authors was that reticulocyte channel can rapidly and efficiently correct the RBC count, without the need to preheat samples with cold agglutinins. Nevertheless, this cannot completely solve the residual presence of cold agglutinins in all samples (45). These observations are in line with the conclusions of several other previously published papers, that evaluated the possibility of adjusting erythrocytes agglutination using the reticulocytes channel of the hematological analyzers (46,47).
Modern analyzers in the diagnosis of myelodysplastic syndrome (MDS)
The modern hematology analyzers automatically provide, new data defined “cell population data” (CPD). These data are only for research purpose.
A very recent application of modern analyzers adopting new CPD parameters was used to identify patients with MDS, among subjects with normal CBC.
Kim et al. showed that CPD representing cell volume (decreased neutrophil forward scatter light) and complexity of neutrophils (decreased neutrophil side scatter light) may be useful for screening MDS using peripheral blood (PB) (48). The existing known markers, low RBC count and platelet count, showed highest diagnostic efficiency in screening MDS rapidly without additional cost (48).
Since the diagnosis of MDS is based on the interpretation of bone marrow morphology, the detection of PB abnormalities in MDS by using an automated analyzer would be extremely helpful (49-51). Unfortunately, a recent publication by Murphy and colleagues on the use of CPD to differentiate patients with MDS and those with other forms of anemia, did not found statistically significant difference in CPD parameters between patients with MDS and those with other normochromic anemias (49). Therefore, more studies are needed to validate CPD data for the diagnosis of MDS patients.
Microbiology and hematological analyzers
Additional information provided by modern hematological analyzers can be very useful for microbiologist. For example, several authors reported cases of Pseudo-erythroblastosis identified with the hematology analyzer caused by Candida sepsis. The presence of Candida albicans blastospores in the PB, determined morphological anomalies on cytogram of modern hematological analyzers provided by different companies, extended from the noise to close the nucleated RBCs zone, generating a result of Pseudo-erythroblastosis (52-56). These represent other practical example of how “zero-cost data” might be useful, once correctly interpreted, for the identification of fungal infection. The clinical repercussions on the management of the patient are therefore relevant.
In keeping with this, the presence of Toxoplasma in the PB was recently identified by a hematological analyzer, thanks to the alarm related to the presence of erythroblasts (57). The scattergram related to the channel WNR (WBC and nucleated red blood cells) showed an abnormal cluster in the zone normally associated with erythroblasts. In this case, the cluster was generated by the presence of Toxoplasma, subsequently confirmed by microscopic examination (57).
Similarly, Roccaforte et al. described an interesting case of unexpected diagnosis of Plasmodium falciparum. The investigation was triggered because an abnormal cellular cluster in the scattergram relative to the white blood cell differential (WDF) channel of blood count on XN-analyzer (53). Optical microscopy was performed and showed that the abnormal cluster was consistent with parasitized blood cells (58).
This study confirms previous results obtained with the different hematological analyzers from Beckmann-Coulter and Abbott, and also underlines the possibility to identify subjects with malaria thanks to the abnormalities observed in cellular scattergrams provided by different hematological analyzers (59-62).
Inflammation or infections
Modern hematology analyzers can also efficiently differentiate between inflammation and infection. Changes in neutrophils, lymphocytes and monocytes are observed in the course of infection and inflammation. However, identifying these morphological changes is often complex and laborious. In 2011, Park et al. determined the utility of leukocyte CPD for the screening of sepsis and fungemia using Beckman Coulter DxH800TM. The authors showed that many of the CPDs of neutrophils, lymphocytes, and monocytes can be considered useful sepsis-parameters. These parameters can be incorporated into a decision rule for the screening of sepsis samples and to discriminate fungemia from bacteremia (63). Very recently, Aguirre et al. reported the diagnostic performance of a machine learning model using CPD provided by the Mindray BC-6800-PlusTM analyzer for the detection of sepsis. The author’s conclusion was that CPD data can be useful at patient admission time for establishing a cost-effective sepsis prediction (64). Urrechaga et al. explored the leukocyte differential and CPD parameters reported by the Mindray BC-6800-PlusTM analyzer in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and those suffering from infections of different etiologies admitted to the emergency department. The conclusion of these authors was that among CPD, the neutrophils-derived parameters are useful for the discrimination between SARS-CoV-2 and other infections (65).
Interestingly, after the first cases of coronavirus disease 2019 (COVID-19) several studies used the CPD data for a timely diagnosis of the disease with the intent of monitoring, and to help establish the prognosis and disease severity. However, CPD values largely depend on analyzer type and CPD from different analyzers are not necessarily comparable, hindering the widespread of these parameters (66-70). Therefore, further studies are needed to confirm these early encouraging findings.
Recently, Polilli et al. presented a study on monocyte distribution width (MDW) a parameter provide by the UniCel DxH800TM (Beckman Coulter) instrument, that may play a role in identifying patients with sepsis in comparison with procalcitonin. This study showed how the use of MDW together with routine WBC counts and indices may be of remarkable use to detect sepsis (71).
Conclusions
In conclusion, modern hematological analyzers can provide information that goes beyond just the simple counting and differentiation of blood cells. Laboratory specialists should become aware and familiarise with the potential offered by the current instruments. We believed that if these laboratory data are incorporated into well-designed clinical studies, they would certainly contribute to better clinical patient management.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-32/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seo JY, Lee ST, Kim SH. Performance evaluation of the new hematology analyzer Sysmex XN-series. Int J Lab Hematol 2015;37:155-64. [Crossref] [PubMed]
- Lippi G, Plebani M. Recent developments and innovations in red blood cells diagnostics. J Lab Precis Med 2018;3:68. [Crossref]
- Siekmeier R, Bierlich A, Jaross W. The white blood cell differential: three methods compared. Clin Chem Lab Med 2001;39:432-45. [Crossref] [PubMed]
- Zandecki M, Genevieve F, Gerard J, et al. Spurious counts and spurious results on haematology analysers: a review. Part I: platelets. Int J Lab Hematol 2007;29:4-20. [Crossref] [PubMed]
- Zandecki M, Genevieve F, Gerard J, et al. Spurious counts and spurious results on haematology analysers: a review. Part II: white blood cells, red blood cells, haemoglobin, red cell indices and reticulocytes. Int J Lab Hematol 2007;29:21-41. [Crossref] [PubMed]
- Coulter WH. Means for counting particles suspended in a fluid. United States Patent 2656508. 1949.
- Van Dilla MA, Fulwyler MJ, Boone IU. Volume distribution and separation of normal human leucocytes. Proc Soc Exp Biol Med 1967;125:367-70. [Crossref] [PubMed]
- Gauthier J, Harel P. Human leukocytes: their size distribution and mean corpuscular volume. Can Med Assoc J 1967;97:793-6. [PubMed]
- Okada T. Development and problem of automated Hematology Analyzers. Sysmex Journal International 1999;9:52-7.
- Coulter WH, Hogg WR. Signal modulated apparatus for generating and detecting resistive and reactive changes in a modulated current path for particle classification on analysis. United States Patent 3502974. 1966.
- Leif RC, Thomas RA. Electronic cell-volume analysis by use of the AMAC 1 Transducer. Clin Chem 1973;19:853-70. [Crossref] [PubMed]
- Thomas RA, Yopp TA, Watson BD, et al. Combined optical and electronic analysis of cells with the AMAC transducer. J Histochem Cytochem 1977;25:827-35. [Crossref] [PubMed]
- Hoffman RA, Britt WB. Flow-system measurement of cell impedance properties. J Histochem Cytochem 1979;27:234-40. [Crossref] [PubMed]
- Moldavan A. Photo-electric technique for the counting of microscopical cells. Science 1934;80:188-9. [Crossref] [PubMed]
- Crosland-Taylor PJ. A device for counting small particles suspended in a fluid through a tube. Nature 1953;171:37-8. [Crossref] [PubMed]
- Kamentsky LA, Melamed MR, Derman H. Spectrophotometer: new instrument for ultrarapid cell analysis. Science 1965;150:630-1. [Crossref] [PubMed]
- Kim YR, Ornstein L. Isovolumetric sphering of erythrocytes for more accurate and precise cell volume measurement by flow cytometry. Cytometry 1983;3:419-27. [Crossref] [PubMed]
- Mohandas N, Kim YR, Tycko DH, et al. Accurate and independent measurement of volume and hemoglobin concentration of individual red cells by laser light scattering. Blood 1986;68:506-13. [Crossref] [PubMed]
- Kachel V, Glossner E, Kordwig E, et al. Fluvo-metricell, a combined cell volume and cell fluorescence analyzer. J Histochem Cytochem 1977;25:804-12. [Crossref] [PubMed]
- Kleinerman M. Differential counting of leukocytes and other cells. United States Patent 3916205. 1975.
- Shapiro HM, Schildkraut ER, Curbelo R, et al. Combined blood cell counting and classification with fluorochrome stains and flow instrumentation. J Histochem Cytochem 1976;24:396-401. [Crossref] [PubMed]
- Leif RC, Thomas RA, Yopp TA, et al. Development of instrumentation and fluorochromes for automated multiparameter analysis of cells. Clin Chem 1977;23:1492-8. [Crossref] [PubMed]
- Pati AR, Patton WN, Harris RI. The use of the technicon H1 in the diagnosis of hereditary spherocytosis. Clin Lab Haematol 1989;11:27-30. [Crossref] [PubMed]
- Sugandha Kakkar N, John JM. Utility of hyperchromic cell percentage, flags, and red cell cytograms generated by Advia-120 hematology analyzer as a potential screening tool in hereditary spherocytosis. J Hematop 2021;14:137-43. [Crossref]
- Rooney S, Hoffmann JJ, Cormack OM, et al. Screening and confirmation of hereditary spherocytosis in children using a CELL-DYN Sapphire haematology analyser. Int J Lab Hematol 2015;37:98-104. [Crossref] [PubMed]
- Nivaggioni V, Bouriche L, Coito S, et al. Use of Sysmex XN-10 red blood cell parameters for screening of hereditary red blood cell diseases and iron deficiency anaemia. Int J Lab Hematol 2020;42:697-704. [Crossref] [PubMed]
- Nivaggioni V, Van Mirre E, Brousseau J, et al. Detection of Southern Asian Ovalocytosis with Sysmex XN-10: A complement to the decision tree previously described. Int J Lab Hematol 2022;44:e84-6. [Crossref] [PubMed]
- Mullier F, Lainey E, Fenneteau O, et al. Additional erythrocytic and reticulocytic parameters helpful for diagnosis of hereditary spherocytosis: results of a multicentre study. Ann Hematol 2011;90:759-68. [Crossref] [PubMed]
- King MJ, Garçon L, Hoyer JD, et al. ICSH guidelines for the laboratory diagnosis of nonimmune hereditary red cell membrane disorders. Int J Lab Hematol 2015;37:304-25. [Crossref] [PubMed]
- Persijn L, Bonroy C, Mondelaers V, et al. Screening for hereditary spherocytosis in routine practice: evaluation of a diagnostic algorithm with focus on non-splenectomised patients. Ann Hematol 2012;91:301-2. [Crossref] [PubMed]
- Sottiaux JY, Favresse J, Chevalier C, et al. Evaluation of a hereditary spherocytosis screening algorithm by automated blood count using reticulocytes and erythrocytic parameters on the Sysmex XN-series. Int J Lab Hematol 2020;42:e88-91. [Crossref] [PubMed]
- Bobée V, Daliphard S, Schrapp A, et al. Screening of hereditary spherocytosis and pyruvate kinase deficiency by automated blood count using erythrocytic and reticulocytic parameters. Int J Lab Hematol 2018;40:697-703. [Crossref] [PubMed]
- Hoffmann JJ, Urrechaga E, Aguirre U. Discriminant indices for distinguishing thalassemia and iron deficiency in patients with microcytic anemia: a meta-analysis. Clin Chem Lab Med 2015;53:1883-94. [Crossref] [PubMed]
- Urrechaga E, Borque L, Escanero JF. The role of automated measurement of RBC subpopulations in differential diagnosis of microcytic anemia and β-thalassemia screening. Am J Clin Pathol 2011;135:374-9. [Crossref] [PubMed]
- Urrechaga E. Discriminant value of % microcytic/% hypochromic ratio in the differential diagnosis of microcytic anemia. Clin Chem Lab Med 2008;46:1752-8. [Crossref] [PubMed]
- Urrechaga E. The new mature red cell parameter, low haemoglobin density of the Beckman-Coulter LH750: clinical utility in the diagnosis of iron deficiency. Int J Lab Hematol 2010;32:e144-50. [Crossref] [PubMed]
- Ng EH, Leung JH, Lau YS, et al. Evaluation of the new red cell parameters on Beckman Coulter DxH800 in distinguishing iron deficiency anaemia from thalassaemia trait. Int J Lab Hematol 2015;37:199-207. [Crossref] [PubMed]
- Schoorl M, Schoorl M, Linssen J, et al. Efficacy of advanced discriminating algorithms for screening on iron-deficiency anemia and β-thalassemia trait: a multicenter evaluation. Am J Clin Pathol 2012;138:300-4. [Crossref] [PubMed]
- Roccaforte V, Daves M, Lippi G, et al. Preliminary assessment of the new Sysmex XN parameter Iron-Def for identifying iron deficiency. Blood Transfus 2020;18:406-12. [PubMed]
- Bakri NA, Mohd Tohit ER, Idris F, et al. Assessment of reticulocyte-haemoglobin equivalent (Ret-He) as a diagnostic indicator for iron deficiency anaemia. Malaysian Journal of Medicine and Health Sciences 2020;16:58-63.
- Buttarello M. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol 2016;38:123-32. [Crossref] [PubMed]
- Urrechaga E, Hoffmann JJML. Assessment of iron-restricted erythropoiesis in chronic renal disease: evaluation of Abbott CELL-DYN Sapphire mean reticulocyte hemoglobin content (MCHr). Scand J Clin Lab Invest 2019;79:363-7. [Crossref] [PubMed]
- Fletcher A, Forbes A, Svenson N, et al. Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children. Br J Haematol 2022;196:523-9. [Crossref] [PubMed]
- Buttarello M, Rauli A, Mezzapelle G. Reticulocyte count and extended reticulocyte parameters by Mindray BC-6800: Reference intervals and comparison with Sysmex XE-5000. Int J Lab Hematol 2017;39:596-603. [Crossref] [PubMed]
- Roccaforte V, Sciarini F, Proserpio V, et al. Use of the reticulocyte channel warmed to 41°C of the XN-9000 analyzer in samples with the presence of cold agglutinins. Hematol Transfus Cell Ther 2021;43:147-55. [Crossref] [PubMed]
- La Gioia A, Fumi M, Fiorini F, et al. Short preheating at 41°C leads to a red blood cells count comparable to that in RET channel of Sysmex analysers in samples showing cold agglutination. J Clin Pathol 2018;71:729-34. [Crossref] [PubMed]
- Falvella FS, Serafini L, Birindelli S, et al. Validation of the reticulocyte channel of Sysmex XN-9000 system for blood cell count in samples with suspected cold agglutination for use in a total laboratory automation setting. J Clin Pathol 2020;73:847-8. [Crossref] [PubMed]
- Kim H, Han E, Lee HK, et al. Screening of myelodysplastic syndrome using cell population data obtained from an automatic hematology analyzer. Int J Lab Hematol 2021;43:e54-7. [Crossref] [PubMed]
- Murphy PT, Bergin S, O’Brien M, et al. Cell population data from Sysmex XN analyzer and myelodysplastic syndrome. Int J Lab Hematol 2022;44:e138-9. [Crossref] [PubMed]
- Hwang SM, Nam Y. Complete blood count and cell population data parameters from the Abbott Alinity hq analyzer are useful in differentiating myelodysplastic syndromes from other forms of cytopenia. Int J Lab Hematol 2022;44:468-76. [Crossref] [PubMed]
- Furundarena JR, Araiz M, Uranga M, et al. The utility of the Sysmex XE-2100 analyzer’s NEUT-X and NEUT-Y parameters for detecting neutrophil dysplasia in myelodysplastic syndromes. Int J Lab Hematol 2010;32:360-6. [Crossref] [PubMed]
- La Gioia A, Basile M, Fiorini F, et al. Pseudo-erythroblastosis on Sysmex XN hematology analyzers: a clue to Candida sepsis. Case report and literature review. Clin Chem Lab Med 2022;60:e210-2. [Crossref] [PubMed]
- La Gioia A, Bombara M, Fiorini F, et al. Earlier detection of sepsis by Candida parapsilosis using three-dimensional cytographic anomalies on the Mindray BC-6800 hematological analyzer. Clin Chem Lab Med 2016;54:e239-42. [Crossref] [PubMed]
- La Gioia A, Devito A, Fiorini F, et al. Cytographic changes on BC-6800 Haematological Analyzer related to the presence of Candida albicans in peripheral blood. A new tool to suspect candidemia? J Clin Pathol 2017;70:494-9. [Crossref] [PubMed]
- Gérard D, Lesesve JF. Abnormal dot plots on current automated blood cell analyzer helped to yeast detection. Clin Case Rep 2020;8:776-7. [Crossref] [PubMed]
- Comar SR, Spiri BS, Ferreira DS, et al. Early detection of Candida parapsilosis sepsis in peripheral blood as a result of cytografic changes on the Sysmex XN-3000 hematology analyzer. Int J Lab Hematol 2021;43:e280-3. [Crossref] [PubMed]
- Daves M, Roccaforte V, Jani E, et al. The unexpected erythroblast: a case report. Riv Ital Med Lab 2019;15:300-2.
- Roccaforte V, Liuzzi G, Porreca WP, et al. A case of malaria diagnosed accidentally with Sysmex XN-9000 automated analyzer. J Lab Precis Med 2019;4:13. [Crossref]
- Campuzano-Zuluaga G, Alvarez-Sánchez G, Escobar-Gallo GE, et al. Design of malaria diagnostic criteria for the Sysmex XE-2100 hematology analyzer. Am J Trop Med Hyg 2010;82:402-11. [Crossref] [PubMed]
- Huh HJ, Oh GY, Huh JW, et al. Malaria detection with the Sysmex XE-2100 hematology analyzer using pseudoeosinophilia and abnormal WBC scattergram. Ann Hematol 2008;87:755-9. [Crossref] [PubMed]
- Mohapatra S, Samantaray JC, Arulselvi S, et al. Automated detection of malaria with haematology analyzer Sysmex XE-2100. Indian J Med Sci 2011;65:26-31. [Crossref] [PubMed]
- Campuzano-Zuluaga G, Hänscheid T, Grobusch MP. Automated haematology analysis to diagnose malaria. Malar J 2010;9:346. [Crossref] [PubMed]
- Park DH, Park K, Park J, et al. Screening of sepsis using leukocyte cell population data from the Coulter automatic blood cell analyzer DxH800. Int J Lab Hematol 2011;33:391-9. [Crossref] [PubMed]
- Aguirre U, Urrechaga E. Diagnostic performance of machine learning models using cell population data for the detection of sepsis: a comparative study. Clin Chem Lab Med 2023;61:356-65. [Crossref] [PubMed]
- Urrechaga E, Ponga C, Fernández M, et al. Diagnostic potential of leukocyte differential and cell population data in prediction of COVID-19 among related viral and bacterial infections at Emergency Department. Clin Chem Lab Med 2022;60:e104-7. [Crossref] [PubMed]
- Martens RJH, van Adrichem AJ, Mattheij NJA, et al. Hemocytometric characteristics of COVID-19 patients with and without cytokine storm syndrome on the sysmex XN-10 hematology analyzer. Clin Chem Lab Med 2020;59:783-93. [Crossref] [PubMed]
- Urrechaga E, Aguirre U, España PP, et al. Complete blood counts and cell population data from Sysmex XN analyser in the detection of SARS-CoV-2 infection. Clin Chem Lab Med 2020;59:e57-60. [Crossref] [PubMed]
- Lapić I, Brenčić T, Rogić D, et al. Cell population data: Could a routine hematology analyzer aid in the differential diagnosis of COVID-19? Int J Lab Hematol 2021;43:e64-7. [Crossref] [PubMed]
- Harte JV, Mykytiv V. A panhaemocytometric approach to COVID-19: a retrospective study on the importance of monocyte and neutrophil population data on Sysmex XN-series analysers. Clin Chem Lab Med 2021;59:e169-72. [Crossref] [PubMed]
- Martens RJH, Leers MPG. Letter in reply to the letter to the editor of Harte JV and Mykytiv V with the title “A panhaemocytometric approach to COVID-19: a retrospective study on the importance of monocyte and neutrophil population data”. Clin Chem Lab Med 2021;59:e173-4. [Crossref] [PubMed]
- Polilli E, Sozio F, Frattari A, et al. Comparison of Monocyte Distribution Width (MDW) and Procalcitonin for early recognition of sepsis. PLoS One 2020;15:e0227300. [Crossref] [PubMed]
Cite this article as: Daves M, Roccaforte V, Lombardi F, Panella R, Pastori S, Spreafico M, Jani E, Piccin A. Modern hematology analyzers: beyond the simple blood cells count (with focus on the red blood cells). J Lab Precis Med 2024;9:4.