
Cardiac troponin and the diagnosis of type 2 myocardial infarction and acute non-ischaemic myocardial injury
Introduction
Cardiac troponin is the only recommended biomarker for the diagnosis of myocardial infarction and is utilised widely in clinical practice (1). Current high-sensitivity cardiac troponin (hs-cTn) assays are defined by the ability to detect circulating troponin in the majority of healthy individuals with precision (2,3). However, this increased sensitivity has led to a reduction in specificity for the diagnosis of myocardial infarction. Increasingly, we recognize cardiac troponin concentration above the 99th upper reference limit (URL) across a spectrum of both cardiac and non-cardiac pathologies.
Diagnosis of myocardial infarction and myocardial injury
The Universal Definition of Myocardial Infarction (UDMI) was introduced to encourage consensus and in recognition that myocardial infarction may occur due to a variety of underlying pathology (4-6) (Figure 1). The diagnosis of myocardial infarction is applied when an acute rise and/or fall in cardiac troponin elevation is detected, in conjunction with ischaemic symptoms, myocardial ischaemia on the 12-lead electrocardiogram or imaging evidence of a regional wall motion abnormality is identified. A type 1 myocardial infarction occurs due to atherosclerotic plaque rupture, intracoronary thrombosis, subtotal or complete occlusion with distal hypoperfusion causing ischaemia and then irreversible cell necrosis. It is the most common cause of myocardial infarction and where the majority of our evidence for practice exists (7-11).
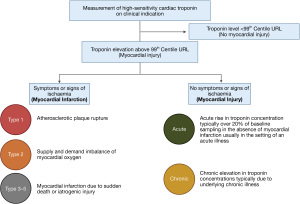
Type 2 myocardial infarction is a descriptive term encompassing patients with a reduction in myocardial oxygen supply or an unmet need in myocardial oxygen demand, without atherothrombosis, and is responsible for approximately one in every five events. Just one third of patients are alive at five years after diagnosis, but to date we have no evidence from prospective randomised controlled trials to guide investigation or treatment (8,12,13). Additional types of myocardial infarction related to sudden cardiac death (type 3), percutaneous coronary intervention (PCI; type 4) or coronary artery bypass graft surgery (CABG; type 5) are also defined, but the evidence to support the suggested diagnostic criteria is uncertain. The definition of type 4 myocardial infarction includes an arbitrary rise of >5 fold greater than the 99th centile with imaging evidence of regional wall motion abnormality, whereas the definition of type 5 myocardial infarction includes a rise of >10 fold greater than the 99th centile (4). These definitions are particularly controversial as they have had a significant impact on the outcome of trials comparing PCI or CABG in patients with left main stem disease (14) (Figure 1).
In patients with an acute rise or fall in cardiac troponin without symptoms or signs of myocardial ischaemia, the diagnosis of acute non-ischaemic myocardial injury is applied. Where there is no dynamic change on serial testing this is classified as chronic myocardial injury (4).
Identifying and correcting the aetiology of supply or demand imbalance in patients with type 2 myocardial infarction or acute non-ischaemic myocardial injury is the principal recommendation for treatment, and several pragmatic approaches have been recommended (15-17).
Mechanisms of troponin release in type 2 myocardial infarction and non-ischaemic myocardial injury
In patients with type 1 myocardial infarction, subtotal or complete epicardial coronary occlusion leads to tissue hypoxia, ischaemia, infarction and necrosis due to cell membrane lysis (18). Troponin is released in a time dependent manner, peaking approximately 12 hours after injury. Peak troponin concentrations are consistently higher in patients with type 1 myocardial infarction than type 2 myocardial infarction or myocardial injury (8,19,20) (Figure 2).
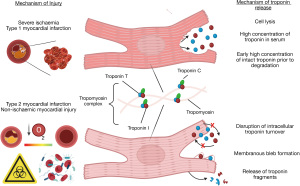
Type 2 myocardial infarction may occur in patients with fixed obstructive coronary artery disease, or in those without obstructive coronary artery disease in the context of vasospasm, coronary embolism or dissection. Some patients with type 2 myocardial infarction and acute non-ischaemic myocardial injury have normal coronary arteries. Mechanisms of myocardial injury and cardiac troponin release are poorly understood. Where there is complete vessel occlusion due to vasospasm, embolism or dissection, the clinical presentation is similar to type 1 myocardial infarction, with ischaemia, infarction and likely cardiomyocyte necrosis. However, in patients without coronary artery occlusion who have a stress response to physiological insult such as tachyarrhythmia, hypoxemia or hypotension, troponin release from the cardiomyocyte may occur due to cytosolic leak without membrane rupture, disruption of normal intracellular turnover or membranous bleb formation (21-24) (Figure 2). Recently troponin was detected enriched in circulating extracellular vesicles in patients with documented unstable angina. This may serve as a future biomarker to risk stratify patients with both acute and chronic coronary syndromes for intensification of cardioprotective therapies (25,26).
Using cardiac troponin to differentiate myocardial injury or infarction subtype
A secondary analysis of the high-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome (High-STEACS) trial revealed that troponin I concentrations were higher, with a greater absolute and relative change in patients with type 1 compared to type 2 myocardial infarction and acute non-ischaemic myocardial injury (27). This has also been demonstrated also in a pooled analysis of six clinical trials for both troponin T and I assays (28). However, when using presentation concentration in combination with a relative change of >20% to predict a diagnosis of type 1 myocardial infarction, discrimination was only moderate, with an area under the receiving operator curve (AUC) of 0.66 [95% confidence interval (CI): 0.64 to 0.68] (27). Cardiac troponin alone cannot differentiate myocardial injury or infarction subtypes and should never guide diagnosis in isolation (27,29).
Using cardiac troponin to risk stratify type 2 myocardial infarction and acute myocardial injury
Traditional risk stratification tools developed in type 1 myocardial infarction such as the GRACE 2.0 or TIMI risk score incorporate cardiac biomarkers, with a binary decision threshold above or below the assay specific URL (30,31). These scores predate the implementation of hs-cTn and the Universal Definition, but even the latest iteration of GRACE (3.0) fails to take advantage of the enhanced precision and clinical risk information provided by use of peak concentrations as a continuous variable.
The GRACE 2.0 algorithm has been evaluated in patients with type 2 myocardial infarction, where modest discrimination for myocardial infarction or death was observed (AUC =0.70, 95% CI: 0.6–0.74) (32). More bespoke risk prediction algorithms have now been developed. The Troponin Assessment for Risk stRatification of patients without Acute COronary atherothrombosis (TARRACO) risk score was developed in patients with acute non-ischaemic myocardial injury and type 2 myocardial infarction and predicts a primary outcome of either death, myocardial infarction or heart failure rehospitalisation at 180 days, with moderate discrimination (AUC =0.74, 95% CI: 0.70–0.79). This tool uses an arbitrary value of >5× the 99th centile URL, with an adjusted odds ratio of 1.46 (95% CI: 0.92–2.32) for the primary outcome (33). This score did not validate as well in a further independent analysis, albeit it is accepted a different endpoint was evaluated which did not include heart failure hospitalisation.
The T2-risk score is a novel score prospectively derived and validated in consecutive patients with type 2 myocardial infarction from the High-STEACS trial and validated in two populations; the APACE trial of non-consecutive patients with suspected acute coronary syndrome, and consecutive patients from the Karolinska Institute. This incorporates cardiac troponin I as a continuous variable as well as clinically relevant co-variates including age, estimated glomerular filtration rate (eGFR), and heart rate, the presence of ischaemic heart disease, anaemia, previous heart failure hospitalisation or ischaemia on the electrocardiogram. This score predicted all-cause death or myocardial infarction at one year with moderate discrimination (AUC =0.76, 95% CI: 0.73–0.79) and demonstrated an adjusted multivariable hazard ratio for the primary outcome of 1.32 (95% CI: 1.12–1.55) (34).
Randomised controlled trials in type 2 myocardial infarction and acute myocardial injury
Despite type 2 myocardial infarction and non-ischaemic myocardial injury being recognised for over 15 years, to date no randomised controlled trials have reported to guide investigation or treatment. Patients with type 2 myocardial infarction and acute non-ischaemic myocardial injury are heterogeneous by definition and present due to a variety of other systemic illnesses (5,13,35,36). This increases complexity when designing interventions which could plausibly improve outcomes.
A recent systematic review was undertaken to identify clinical studies in patients with type 2 myocardial infarction to inform the design and delivery of a Delphi study (37). This review identified two randomised controlled trials and five observational cohort studies currently or recently completed enrolment of patients with type 2 myocardial infarction or acute myocardial injury (Table 1).
Table 1
| Study name | Study type | Number of patients aimed to be recruited | Number of patients actually or currently* recruited | Status | Outcome |
|---|---|---|---|---|---|
| TARGET-Type 2 (38) | A pilot randomised controlled trial of a complex intervention | 60 | 34* | Active | Pending |
| Determining the Mechanism of Myocardial Injury and Role of Coronary Disease in Type 2 Myocardial Infarction (DEMAND-MI) (39) | Prospective observational | 100 | 100 | Completed | Patients with type 2 myocardial infarction incidence of 68% underlying coronary disease and 42% structural heart disease. 7% misclassification rate |
| The appropriateness of coronary investigation in myocardial injury and type 2 myocardial infarction (ACT-2) (40) | Prospective, open-labelled, parallel clustered, randomized controlled trial with blinded end point assessment | 1,800 | Unknown* | Active | Pending |
| DEFINing the PrEvalence and Characteristics of Coronary Artery Disease Among Patients With TYPE 2 Myocardial Infarction Using CT-FFR (DEFINE TYPE2MI) (41) | Prospective observational | 50 | 50 | Completed | Pending |
| A Study of Microcirculatory Function in Type 2 Myocardial Infarction (42) | Observational case control | 52 | 0 | Pre-recruitment phase | Pending |
| Inflammation in Type 2 Myocardial Infarction (43) | Prospective observational | 30 | Unknown | Withdrawn | Withdrawn due to feasibility |
| Rivaroxaban in Type 2 Myocardial Infarctions (R2MI) (44) | A feasibility, placebo-controlled, double-blinded, randomized controlled trial | 100 | 8 | Completed | Unable to identify recruit and randomise over time period |
*, signifies ongoing recruitment.
The DEMAND-MI study recruited 100 patients with a clinical diagnosis of type 2 myocardial infarction and aimed to determine the prevalence of obstructive coronary artery disease and left ventricular systolic dysfunction. This study used invasive or CT coronary angiography and cardiac MRI or echocardiography where appropriate. A high prevalence of underlying coronary heart disease was identified (68%, 63/93), which was obstructive in one third of patients, and evidence of structural heart disease was observed in 42% (39/93). Importantly, these conditions were previously unrecognised in 50% of participants. In a subsequent prospective cohort study with CT coronary angiography and non-invasive fractional flow reserve assessment of consecutive patients with type 2 myocardial infarction, coronary artery disease was identified in 92% of patients, which was obstructive in 42%, and previously unrecognised and untreated in 90% (41,45). This highlights the opportunity to identify and treat previously unrecognised pathology which has manifest in the context of an alternative physiological stressor (39).
It is recognised that recruitment of patients with type 2 myocardial infarction and acute non-ischaemic myocardial injury may be challenging (43,44). One trial attempted to evaluate rivaroxoban using student led recruitment and randomisation, and due to a combination of limited staff time and strict exclusion criteria, closed the study prior to enrolment reaching 10%. However, other trials have had greater success (44).
The TARGET-Type 2 trial is a multi-centre prospective randomised controlled trial evaluating the feasibility of evaluating a complex intervention of investigation and treatment for coronary artery disease or left ventricular dysfunction. To date, over 90% of the intended participants have been enrolled, and this study is due to report on schedule in June 2024. Ultimately, this study will guide the design of a multi-centre randomised controlled trial powered for clinically relevant endpoints (38).
Novel cardiac biomarkers
Whilst cardiac troponin is a specific biomarker of myocardial injury, it is not specific for myocardial infarction. If a novel approach could identify patients with atherothrombotic type 1 myocardial infarction alone, this could be transformative for care providers in guiding immediate investigation and treatment in what are often challenging clinical scenarios.
It is known that cardiac troponin I and cardiac troponin T are expressed in a 1:1 ratio in human myocardium (46), yet peak concentrations of cardiac troponin I are often ten-fold higher and return to normal more promptly than cardiac troponin T after myocardial infarction (47). Following cardiomyocyte necrosis, cardiac troponin I is cleaved promptly and released into the circulation (48). The majority of cardiac troponin T remains bound to cardiomyocyte filaments which undergo local phagocytosis and degradation (49). Therefore, a relatively lower concentration of cardiac troponin T is measurable in circulating plasma (50). Both hs-cTnI and cardiac troponin T assays may detect the intact and fragmented cardiac troponin protein forms, which may influence measurable concentrations, particularly early after injury. No significant differences have been observed in cardiac troponin I and T clearance which occurs through both renal and hepatic mechanisms with similar kinetics.
This difference in cardiac troponin I and T release has been exploited in discriminating myocardial injury from infarction. Across five observational cohort studies, 888 of 3,124 patients with suspected acute coronary syndrome had an adjudicated diagnosis of type 1 (n=408) or type 2 myocardial infarction (n=56) or acute non ischaemic myocardial injury (n=424). The ratio of hs-cTnI to hs-cTnT differed considerably by subtype; highest in type 1 myocardial infarction at 3.45 (1.80–6.59), with type 2 myocardial infarction (1.18, 95% CI: 0.81–1.90) and acute non-ischaemic myocardial injury (0.67, 95% CI: 0.39–1.12) significantly lower. Overall, the hs-cTnI/hs-cTnT ratio provided excellent discrimination with an AUC of 0.89 (95% CI: 0.86–0.91). A ratio of >1.40 gave a specificity and positive predictive value of 80% and 78.5% (95% CI: 74.4–82%), with a ratio of >2.24 giving a specificity and positive predictive value of 90% and 85% (95% CI: 80.7–88.8%), respectively (47). This approach requires prospective validation including cohorts with higher numbers of participants with type 2 myocardial infarction.
There may be other reasons for differences in the profile of cardiac troponin I and T in vivo. It is recognised that heavily cleaved small fragments of cardiac troponin T are detectable in the circulation of patients with chronic renal impairment with high sensitivity assays (51). A novel cardiac troponin T assay has been developed which uses a capture antibody binding an epitope adjacent to the C-terminus, with further antibodies binding the central portion of the troponin molecule to identify intact cardiac troponin T (52). A higher ratio of long-form cardiac troponin T to total cardiac troponin T was observed in 117 patients with myocardial infarction when compared to 41 patients with chronic myocardial injury due to end stage renal failure. In patients with non-ST segment elevation myocardial infarction presenting within 24 hours of pain onset, despite total cardiac troponin T concentrations being comparable with the chronic myocardial injury group, the AUC for the novel full troponin assay was 0.96 (95% CI: 0.89–1.00) (51). This approach holds major promise in differentiating myocardial injury from infarction, with studies of release kinetics and an evaluation in different patient groups warranted.
Cellular necrosis may be detected through the identification of fragments of genomic DNA which are released and briefly circulate in the bloodstream prior to hepatic clearance. A cardiomyocyte specific cell-free DNA (cfDNA) assay has been developed which targets the FAM101A locus (53). In patients with STEMI this assay had high diagnostic accuracy, with an AUC of 0.94 (95% CI: 0.91–0.98) compared to healthy controls. Interestingly, this assay identified an additional one in six patients with STEMI who had apparently normal cardiac troponin concentrations as having cardiomyocyte necrosis. Importantly, cardiomyocyte necrosis was also detectable in a population of patients who were in critical care for sepsis. Whilst total cfDNA concentrations were high, the cardiomyocyte specific cfDNA concentration was comparable to patients with STEMI (54). Further studies are required to evaluate cardiomyocyte specific cfDNA in patients with acute non-ischaemic myocardial injury and type 2 myocardial infarction where serial samples are obtained.
Future directions for research
The combination of an increase in the sensitivity of cardiac biomarkers in an aging co-morbid population has led to an increase in the recognition of type 2 myocardial infarction and acute non-ischaemic myocardial injury in clinical practice. Whilst these diagnoses are associated with poor clinical outcomes, we have no proven strategies for investigation or treatment shown to modify outcomes. Indeed, there is major uncertainty as to whether outcomes are modifiable given the high prevalence of frailty and co-morbidity. These patients are often unwell with multiorgan dysfunction which may lead to challenges in the evaluation of therapies with the potential for iatrogenic harm. However, rates of future myocardial infarction or cardiovascular death are as high as one in six at one year. Future clinical trials should focus on improving the accuracy of clinical diagnosis with novel biomarker approaches, defining and testing strategies for risk stratification and further investigation, and treating underlying coronary or structural heart disease with evidence-based therapies. This approach is likely to have the greatest impact on future cardiovascular outcomes in the short to medium term.
Conclusions
Following the implementation of hs-cTn assays, both acute non-ischaemic myocardial injury and type 2 myocardial infarction are increasingly recognised in practice. In an increasingly elderly and co-morbid population, the prevalence of these conditions is likely to increase further in the coming years. Current treatment strategies focus on correcting the mechanism of supply or demand imbalance, but evidence is emerging that important unaddressed cardiovascular disease exists. Targeted investigation for coronary disease and left ventricular impairment followed by appropriate secondary prevention therapy may provide the best opportunity to modify future cardiovascular risk. It is now time that such strategies are evaluated prospectively in randomised controlled trials.
Acknowledgments
Funding: This work was supported by a British Heart Foundation Clinical Research Training Fellowship (FS/CRTF/21/2473 to C.T.) and a Research Excellence Award (RE/18/5/34216 to A.R.C.).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Xander van Wijk, Amy Saenger, Steven Meex, and Allan Jaffe) for the series “Cardiac Troponin” published in the Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-40/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-23-40/coif). The series “Cardiac Troponin” was commissioned by the editorial office without any funding or sponsorship. C.T. is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/CRTF/21/2473). A.R.C. is supported by a Research Excellence Award (RE/18/5/34216). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anand A, Shah ASV, Beshiri A, et al. Global Adoption of High-Sensitivity Cardiac Troponins and the Universal Definition of Myocardial Infarction. Clin Chem 2019;65:484-9. [Crossref] [PubMed]
- Apple FS, Collinson POIFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61. [Crossref] [PubMed]
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574-81. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237-69. [Crossref] [PubMed]
- Bularga A, Taggart C, Mendusic F, et al. Assessment of Oxygen Supply-Demand Imbalance and Outcomes Among Patients With Type 2 Myocardial Infarction: A Secondary Analysis of the High-STEACS Cluster Randomized Clinical Trial. JAMA Netw Open 2022;5:e2220162. Erratum in: JAMA Netw Open 2023;6:e2314903. [Crossref] [PubMed]
- Gaggin HK, Liu Y, Lyass A, et al. Incident Type 2 Myocardial Infarction in a Cohort of Patients Undergoing Coronary or Peripheral Arterial Angiography. Circulation 2017;135:116-27. [Crossref] [PubMed]
- Shah ASV, Anand A, Strachan FE, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919-28. [Crossref] [PubMed]
- Chapman AR, Adamson PD, Shah ASV, et al. High-Sensitivity Cardiac Troponin and the Universal Definition of Myocardial Infarction. Circulation 2020;141:161-71. [Crossref] [PubMed]
- Roos A, Sartipy U, Ljung R, et al. Relation of Chronic Myocardial Injury and Non-ST-Segment Elevation Myocardial Infarction to Mortality. Am J Cardiol 2018;122:1989-95. [Crossref] [PubMed]
- Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;2:349-60. [PubMed]
- Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993;328:673-9. [Crossref] [PubMed]
- Chapman AR, Shah ASV, Lee KK, et al. Long-Term Outcomes in Patients With Type 2 Myocardial Infarction and Myocardial Injury. Circulation 2018;137:1236-45. [Crossref] [PubMed]
- Lambrecht S, Sarkisian L, Saaby L, et al. Different Causes of Death in Patients with Myocardial Infarction Type 1, Type 2, and Myocardial Injury. Am J Med 2018;131:548-54. [Crossref] [PubMed]
- Stone GW, Kappetein AP, Sabik JF, et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N Engl J Med 2019;381:1820-30. [Crossref] [PubMed]
- DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and Treatment of Patients With Type 2 Myocardial Infarction and Acute Nonischemic Myocardial Injury. Circulation 2019;140:1661-78. [Crossref] [PubMed]
- Chapman AR, Sandoval Y. Type 2 Myocardial Infarction: Evolving Approaches to Diagnosis and Risk-Stratification. Clin Chem 2021;67:61-9. [Crossref] [PubMed]
- Taggart C, Wereski R, Mills NL, et al. Diagnosis, Investigation and Management of Patients with Acute and Chronic Myocardial Injury. J Clin Med 2021;10:2331. [Crossref] [PubMed]
- Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res 2013;113:428-38. [Crossref] [PubMed]
- Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med 2013;126:789-97. [Crossref] [PubMed]
- Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 Myocardial Infarction and Myocardial Injury: Clinical Transition to High-Sensitivity Cardiac Troponin I. Am J Med 2017;130:1431-9.e4. [Crossref] [PubMed]
- Hammarsten O, Mair J, Möckel M, et al. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018;23:725-34. [Crossref] [PubMed]
- Wu AHB. Medicine Release of cardiac troponin from healthy and damaged myocardium. Front Lab Med 2017;1:144-50. [Crossref]
- White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol 2011;57:2406-8. Correction appears in J Am Coll Cardiol 2011;58:2356.
- Fridén V, Starnberg K, Muslimovic A, et al. Clearance of cardiac troponin T with and without kidney function. Clin Biochem 2017;50:468-74. [Crossref] [PubMed]
- D'Ascenzo F, Femminò S, Ravera F, et al. Extracellular vesicles from patients with Acute Coronary Syndrome impact on ischemia-reperfusion injury. Pharmacol Res 2021;170:105715. [Crossref] [PubMed]
- Femminò S, D'Ascenzo F, Ravera F, et al. Percutaneous Coronary Intervention (PCI) Reprograms Circulating Extracellular Vesicles from ACS Patients Impairing Their Cardio-Protective Properties. Int J Mol Sci 2021;22:10270. [Crossref] [PubMed]
- Wereski R, Kimenai DM, Taggart C, et al. Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction. Circulation 2021;144:528-38. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Cervellin G. Cardiac troponins and mortality in type 1 and 2 myocardial infarction. Clin Chem Lab Med 2017;55:181-8. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Cervellin G. Chest pain, dyspnea and other symptoms in patients with type 1 and 2 myocardial infarction. A literature review. Int J Cardiol 2016;215:20-2. [Crossref] [PubMed]
- Fox KA, Fitzgerald G, Puymirat E, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014;4:e004425. [Crossref] [PubMed]
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000;284:835-842. [Crossref] [PubMed]
- Hung J, Roos A, Kadesjö E, et al. Performance of the GRACE 2.0 score in patients with type 1 and type 2 myocardial infarction. Eur Heart J 2021;42:2552-61. [Crossref] [PubMed]
- Cediel G, Sandoval Y, Sexter A, et al. Risk Estimation in Type 2 Myocardial Infarction and Myocardial Injury: The TARRACO Risk Score. Am J Med 2019;132:217-26. [Crossref] [PubMed]
- Taggart C, Monterrubio-Gómez K, Roos A, et al. Improving Risk Stratification for Patients With Type 2 Myocardial Infarction. J Am Coll Cardiol 2023;81:156-68. [Crossref] [PubMed]
- Kadesjö E, Roos A, Siddiqui A, et al. Acute versus chronic myocardial injury and long-term outcomes. Heart 2019;105:1905-12. [Crossref] [PubMed]
- Sarkisian L, Saaby L, Poulsen TS, et al. Clinical Characteristics and Outcomes of Patients with Myocardial Infarction, Myocardial Injury, and Nonelevated Troponins. Am J Med 2016;129:446.e5-446.e21. [Crossref] [PubMed]
- Taggart C, Ferry A, Chapman A, et al. Consensus on the diagnosis and management of patients with type 2 myocardial infarction: an international delphi study. Heart 2023;109: Abstract.
- Targeting Investigation and Treatment in Patients With Type 2 Myocardial Infarction (TARGET-Type 2) [Internet]. Edinburgh; 2022. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05419583
- Bularga A, Hung J, Daghem M, et al. Coronary Artery and Cardiac Disease in Patients With Type 2 Myocardial Infarction: A Prospective Cohort Study. Circulation 2022;145:1188-200. [Crossref] [PubMed]
- Lambrakis K, French JK, Scott IA, et al. The appropriateness of coronary investigation in myocardial injury and type 2 myocardial infarction (ACT-2): A randomized trial design. Am Heart J 2019;208:11-20. [Crossref] [PubMed]
- Januzzi Jr. JL, Mccarthy CP. DEFINing the PrEvalence and Characteristics of Coronary Artery Disease Among Patients With TYPE 2 Myocardial Infarction Using CT-FFR (DEFINE TYPE2MI) [Internet]. Boston; 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04864119
- A Study of Microcirculatory Function in Type 2 Myocardial Infarction. NCT05793567. Rochester, Minnesota, United States; 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05793567
- Inflammation in Type 2 Myocardial Infarction [Internet]. New York; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02385487
- Gouda P, Kay R, Gupta A, et al. Anticoagulation in type 2 myocardial infarctions: Lessons learned from the rivaroxaban in type 2 myocardial infarctions feasibility trial. Contemp Clin Trials Commun 2023;33:101143. [Crossref] [PubMed]
- McCarthy CP, Murphy SP, Amponsah DK, et al. Coronary Computed Tomographic Angiography With Fractional Flow Reserve in Patients With Type 2 Myocardial Infarction. J Am Coll Cardiol 2023;82:1676-87. [Crossref] [PubMed]
- Potter JD. Preparation of troponin and its subnits. Methods in Enzymology 1982;85:241-63. [Crossref] [PubMed]
- Eggers KM, Hammarsten O, Aldous SJ, et al. Diagnostic and prognostic performance of the ratio between high-sensitivity cardiac troponin I and troponin T in patients with chest pain. PLoS One 2022;17:e0276645. [Crossref] [PubMed]
- Starnberg K, Fridén V, Muslimovic A, et al. A Possible Mechanism behind Faster Clearance and Higher Peak Concentrations of Cardiac Troponin I Compared with Troponin T in Acute Myocardial Infarction. Clin Chem 2020;66:333-41. [Crossref] [PubMed]
- Solecki K, Dupuy AM, Kuster N, et al. Kinetics of high-sensitivity cardiac troponin T or troponin I compared to creatine kinase in patients with revascularized acute myocardial infarction. Clin Chem Lab Med 2015;53:707-14. [Crossref] [PubMed]
- Kragten JA, Hermens WT, van Dieijen-Visser MP. Cardiac troponin T release into plasma after acute myocardial infarction: only fractional recovery compared with enzymes. Ann Clin Biochem 1996;33:314-23. [Crossref] [PubMed]
- Airaksinen KEJ, Aalto R, Hellman T, et al. Novel Troponin Fragmentation Assay to Discriminate Between Troponin Elevations in Acute Myocardial Infarction and End-Stage Renal Disease. Circulation 2022;146:1408-10. [Crossref] [PubMed]
- Park KC, Gaze DC, Collinson PO, et al. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017;113:1708-18. [Crossref] [PubMed]
- Zemmour H, Planer D, Magenheim J, et al. Non-invasive detection of human cardiomyocyte death using methylation patterns of circulating DNA. Nat Commun 2018;9:1443. [Crossref] [PubMed]
- Fridlich O, Peretz A, Fox-Fisher I, et al. Elevated cfDNA after exercise is derived primarily from mature polymorphonuclear neutrophils, with a minor contribution of cardiomyocytes. Cell Rep Med 2023;4:101074. [Crossref] [PubMed]
Cite this article as: Taggart C, Chapman AR. Cardiac troponin and the diagnosis of type 2 myocardial infarction and acute non-ischaemic myocardial injury. J Lab Precis Med 2024;9:5.


