
Investigative algorithms for disorders affecting plasma bilirubin: a narrative review
Introduction
Background
Bilirubin is a product of liver metabolism. Haemoglobin is mostly produced in the bone marrow, with some production in the liver. It contains a central haem moiety, a tetrapyrrole ring (four pyrrole rings consisting of a five-atom ring of four carbon atoms and one nitrogen atom) surrounding a divalent metal ion (iron) which can support gas transport and allows participation in redox reactions, a feature of many other haemoproteins. Commonly high bilirubin concentrations are associated with either an issue with red blood cell turnover, such as haemolysis, or liver function. Distinguishing hyperbilirubinaemia due to a reversible pathology in comparison with a benign phenomenon, such as Gilbert syndrome, can improve patient outcome and guide appropriate management including diagnostic strategy. Low bilirubin concentration is less commonly seen and more likely of low clinical significance. Due to these facts, there is less familiarity with a diagnostic approach to low and therefore if there is a sudden change resulting in concern then it is hoped this section will provide initial guidance on a sensible approach.
Rationale and knowledge gap
Review articles can sometimes be overwhelming for the inexperienced clinician/non-expert clinician if they are not familiar with the subject area. Critical appraisal of the literature and discussion of the minutiae is vital to fully understand what is known and not known on a topic however this can limit utility in clinical practice where some simple guidance is required quickly in regard to the next step. With the increasing variety of healthcare worker backgrounds involved in patient management not all will have had the benefit of a rigorous training in pathology or diagnostics and will gain experience in a more apprentice style model. We aim therefore to provide a series of limited narrative reviews that serve as a starting reference point including easy to use algorithms that can provide such quick ideas to someone presented with a diagnostic conundrum related to abnormal biochemical results. This article should help those who are new to interpreting blood tests and the more familiar reader albeit needing help with an initial framework when seeing an atypical case.
Objective
The following article will discuss the investigation of raised or low bilirubin concentrations for the generalist and present an algorithm to help the reader investigate the cause of elevated bilirubin concentrations. It is not meant to replace specialty guidelines or expertise but is meant to help those who are not sure how to approach an aberrant bilirubin result. We present this article in accordance with the Narrative Review reporting checklist (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-24/rc).
Methods
The narrative review was undertaken with review of Medline, Google Scholar, OMIM and seminal texts. The search was performed from December 2022 to May 2023 to identify references published from database inception to May 2023. Language was restricted to English. Diagnostic algorithms were created from synthesis of the information obtained from literature review. A search strategy summary is outlined in Table S1.
Bilirubin metabolism and regulation
Haem, a ring structure with a metal ion, is ubiquitous in all cells and is integral to the structure of many proteins including haemoglobin, myoglobin and cytochrome enzymes. When broken down the metal iron is recycled, and bilirubin is produced as a by-product, 20% is from non-haemoglobin, the rest from haemoglobin catabolism (Figure 1A) (1,2). Bilirubin, which is water insoluble and unconjugated at this stage, then sequentially:
- Binds to albumin in the plasma (maximum binding capacity of about 20 µmol bilirubin/g albumin (3).
- Is taken up by the liver via organic anion transporting polypeptides (OATP) 1B1 and 3 (facilitates passive uptake into hepatocyte, Figure 1B).
- Binds to glutathione-s-transferase (previously known as Y protein/ligandin) and transported through the hepatocyte.
- In the smooth endoplasmic reticulum is conjugated with glucuronic acid, donated by uridine diphosphate (UDP) sugars and catalysed by the membrane bound enzyme uridine diphosphate glucuronyl transferase 1 (UDP-GT) (4), (process impaired in Crigler Najjar and Gilbert syndrome).
- Water-soluble conjugated bilirubin (mono and diglucoronides) is secreted into the bile tracts via canalicular multi-specific organic anion transporter/multidrug resistance-associated protein 2 (MRP2) (5), (process impaired in Dubin Johnson).
- With other bile components (bile salts/acids, cholesterol, phospholipids, water, electrolytes) and is excreted into the gut lumen (Figure 1A).
- Bacterial processes convert it to stercobilin (the brown pigment in the faeces).
- A minority is further converted to urobilinogen which is reabsorbed in the portal system and excreted in the urine, providing the yellow colour to urine (6).
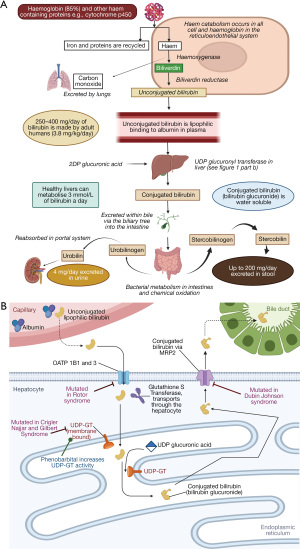
In physiological conditions, bilirubin itself should not be detected in the urine because the unconjugated form is bound to albumin and not filtered in the glomeruli and because the conjugated form is secreted directly into the bowel. If bilirubin is found in the urine, then it is the conjugated form and this is always pathological. Urobilinogen is a normal finding in urine.
The normal bilirubin concentration in plasma is between 2–20 µmol/L. Jaundice can occur at bilirubin concentrations >50 µmol/L but skin pigmentation will affect the clinical sign (7). Unconjugated bilirubin is directly toxic to cells (8-10) and being lipophilic, can cross the blood brain barrier which is why jaundice in infants causes so much concern and can lead to the, sometimes fatal, condition of kernicterus (11). Free unconjugated bilirubin concentrations may rise if albumin is very low, or it is displaced from albumin by drugs such as sulphonamides (covered later), but note free bilirubin is not measured in clinical practice, only unconjugated and conjugated bilirubin can be individually quantified (12).
Measurement methods
Bilirubin is yellow and, unconjugated, has a spectral peak at 450 nm (13). Bilirubin is photosensitive and prolonged light exposure can lead to sample degradation causing spuriously lower concentrations on measurement (14,15). However clinically significant changes in bilirubin are not seen if plasma is left out in the light for up to 8 hours and samples are stable for up to 24 hours when refrigerated without light exposure (16). Fluid bilirubin, e.g., urine, is similarly susceptible to light, and oxidation, and therefore samples are advised to be dipped or analysed immediately (or handled in low or red light and after addition of 1–5 mM ascorbic acid) (17). However, it has been recently demonstrated that there was no significant drift in bilirubin or urobilinogen urine dipstick results in urine samples kept in clear containers at room temperature for up to 4 hours (18).
Commonly the diazo-reaction is used to quantify bilirubin concentration, bilirubin reacts with a diazo reagent to form a coloured azobilirubin. Different accelerants can be used, to promote unconjugated bilirubin to form azobilirubin, and surfactants added to increase solubility (13,19-23). Naproxen metabolites interfere with the diazo-reaction results (24). Paraproteins have been shown to give falsely low bilirubin measurements with the diazo-reaction method (25). Total bilirubin (conjugated and unconjugated) methods are formulated to detect both conjugated and unconjugated bilirubin. In direct methods only conjugated bilirubin is detected via the same method but in the presence of sulfamic acid (20). Indirect (unconjugated) bilirubin therefore is usually a calculated value (total bilirubin minus direct bilirubin, see Figure 2A).
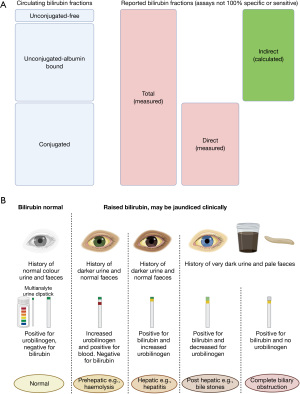
Not all direct/conjugated bilirubin (approximately only 93%) is detected and accuracy may be method dependent (13). If absolute accuracy is required to distinguish unconjugated from conjugated bilirubin then high-pressure liquid chromatography (HPLC) of methyl derivatives is required (26). However HPLC is rarely required as diazo methods are accurate enough to be used clinically.
Alternative methods include bilirubin oxidase (bilirubin converted to biliverdin with the absorption measured at 425 or 460 nm, depending on pH) (analyte methods) and less commonly used is spectrophotometry. Spectrophotometry detects the absorbance of bilirubin at 437 nm and is used in bilirubinometers (transcutaneous devices) and blood gas analysers.
Urine dipsticks can pick up bilirubin and urobilinogen, depending on manufacturer, using diazonium salts to form a red azo dye with the water-soluble conjugated bilirubin or urobilinogen (Figure 2B). If the urine contains highly coloured compounds e.g., phenazopyridine and phenothiazines, indicans, chlorpromazine or etodolac metabolites, then there is a risk of false positive bilirubin results. False negatives are caused by urinary nitrates, acidic urine below pH of 5.5, antibiotic use e.g., rifampicin, old sample (by decreasing intestinal flora) or vitamin C (which will oxidise the bilirubin so it will not react with the strip salts) (27). Urobilinogen is also susceptible, in addition to coloured substances, from interference from amino salicylic acid, sulfonamides and formalin (28).
Urinary urobilinogen on a urine dip is only useful clinically if absent, e.g., if the urine is dark yellow with no urobilinogen (Figure 2B). This likely indicates complete biliary obstruction. In this situation no conjugated bilirubin reaches the gut to form urobilinogen, instead conjugated bilirubin (as being made in the liver but cannot be excreted in the bile) leads to high blood concentrations and hence renal filtration and excretion in the urine. Urine tests should only be performed when specific pathologies are being sought as possible false positives can occur. For example, a study of urine-bilirubin tests in people with normal liver tests demonstrated 0.13% unexpected positive results (29). However, if the urine bilirubin dip is found to be unexpectedly positive 85% of people are found to have an abnormal liver enzyme profile on blood tests performed within 2 weeks after the urine specimen (29).
Bilirubin measurements are inaccurate if the venepuncture process itself causes haemolysis. Blood gas analysers (spectrophotometry) are less affected by haemolysis making it a suitable alternative method e.g., for neonatal bilirubin measurements, or small capillary samples (30-32).
Low bilirubin
Low bilirubin is not usually a clinical concern with some reference intervals including zero and it not being associated with any symptoms. An approach to hypobilirubinaemia, for purely theoretical reasons, is to consider the following situations:
- less bilirubin production;
- increased liver handling;
- increased bilirubin excretion.
Bilirubin concentrations might be a little lower in anaemic patients as there is less haem to be catabolised (33). Drugs can speed up liver metabolism and increase bilirubin degradation including phenobarbital and so can be used as treatments for selected cases of hyperbilirubinaemia (see Table 1 and Figure 1B). The rate limiting step in bilirubin excretion is conjugation in the liver, and not biliary excretion, and so increased stoma output or biliary flow will not affect the bilirubin concentration in plasma.
Table 1
| Drug | Comment |
|---|---|
| Phenobarbital | Enzyme inducing agents; used to treat hereditary hyperbilirubinaemias e.g., Gilbert and Crigler Najjar type 2 |
| Dexamethasone | Enzyme inducing agent* |
| Clofibrate | Enzyme inducing agent* |
| Theophylline and caffeine | By increasing the photodegradation of bilirubin |
| Spironolactone | Enzyme inducing agents* |
| Gluthetimide | Enzyme inducing agents* |
| Rifampicin | Enzyme inducing agents* |
*, induction of uridine diphosphate glucuronyl transferase 1.
High bilirubin
Hyperbilirubinaemia can be approached in different ways, the two commonest approaches are determining whether the bilirubin is conjugated or not, or an anatomical approach, please see the investigative algorithm which uses both approaches respectively (Figure 3A,3B).
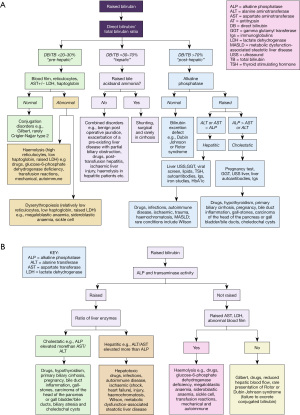
Increased production, pre-hepatic
Haemolytic anaemia is the main cause of an unconjugated hyperbilirubinaemia i.e., pre-hepatic. The breakdown of haem increases the concentration of the unconjugated bilirubin in the plasma (rarely sufficient to cause clinical jaundice) and the liver must increase conjugation and excretion. Urinary urobilinogen may go up and aspartate amino transferase (AST) and lactate dehydrogenase (LDH) will be raised (released from erythrocytes). Haptoglobin concentrations drop (forms a complex with free haem stimulating rapid clearance and iron recycling) and blood films will confirm an anaemia with unusual morphologies (41). Intravascular haemolysis refers to haemolysis that happens in the circulating blood and extravascular to when the red cells are broken down in the reticuloendothelial system, usually the spleen, but often there is a mixed picture (Figure 4). For in depth reviews please see relevant haematology reviews (42,43).
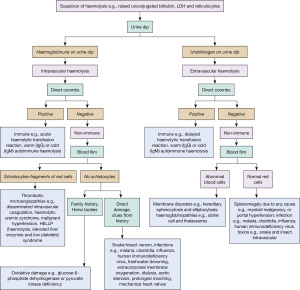
Haemolytic transfusion reactions occur at a rate of approximately 1:70,000 per unit of transfused red blood cells. These can be categorised further as an acute reaction occurring within 24 hours post-transfusion of incompatible red blood cells or a delayed reaction occurring more than 24 hours post-transfusion due to a secondary immune response (44).
Causes of a haemolytic anaemia include:
- Hereditary e.g., sickle cell or glucose-6 phosphate dehydrogenase deficiency;
- Autoimmune acquired e.g., cold agglutinins;
- Drug-induced e.g., dapsone (see Table 2);
- Mechanical stress e.g., mechanical heart valve;
- Paroxysmal nocturnal haemoglobinuria;
- Transfusion reactions.
Table 2
| Mechanism | Examples |
|---|---|
| Haemolysis | Dapsone, sulphasalazine, salazopyrin, sulphonamides, primaquine methyl dopa, menadiol sodium phosphate, methylthionium chloride |
| Inhibit bilirubin conjugation | Alafenamide, disoproxil, emtricitabine, emtricitabine, chloramphenicol, gentamicin, pregnandiol, tryosine kinase inhibitors e.g., imatinib |
| Inhibit biliverdin reductase | MEK and ERK inhibitors e.g., trametinib |
| Hepatoxic | Hydrogenated hydrocarbons e.g., halothane, palonosetron, peginterferon alfa, micafungin, caspofungin, tetracyclines, levofloxacin, nitrofurantoin, isoniazid, penicillins, paracetamol overdose, ethanol, chemotherapeutics e.g., aldesleukin, arsenic trioxide, bexarotene, blinatumomab, bortizumab, busulfan, capecitabine, carfilzomib, clofarabine, cytarabine, eribulin, natalizumab |
| Inhibit bilirubin uptake into hepatocytes | Rifampicin, novobiocin, atanazavir, bictegravir with emtricitabine and tenofovir, elvitegravir with cobicistat, tenofovir, lopinavir and ritonavir, maraviroc, tipranavir, octreotide, contrast media, flavispadic acid |
| Interferes with bilirubin measurement | Eltrombopag |
| Cholestatic | Acetyl choline esterase inhibitors, phenothiazine, imipramine, isavuconazole, itraconazole, thiabendazole, erythromycin, melatonin, oestrogens and anabolic steroids |
| Displace unconjugated bilirubin from albumin | Anti-inflammatory drugs, sulphonamides, cholecystography contrast media, fusidic acid, azapropazone sodium caprylate and N-acetyl tryptophan, sulphonamides and fusidic acid |
MEK, mitogen-activated extracellular signal-regulated kinase; ERK, extracellular regulated protein kinases.
Decreased conjugation/hepatic
Hepatitis and hepatocyte damage will reduce the liver’s ability to conjugate bilirubin and will lead to a rise in both unconjugated and conjugated bilirubin. Elevated liver enzymes, or markers of reduced liver function may be identified along with presence of urinary bilirubin.
An elevation in AST and alanine aminotransferase (ALT) activities, beyond the upper limit of normal (40 and 50 U/L respectively, method dependent) is indicative of hepatic pathology. Other liver enzyme activities may also be elevated in this case including alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) (49).
The causes of liver damage are multiple and include:
- Infections e.g., viral hepatitis;
- Inborn errors of metabolism e.g., haemochromatosis, Wilson, α1-antitrypsin deficiency, glycogen storage disorders, porphyria;
- Autoimmune e.g., autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, systemic lupus erythematosus;
- Ischaemic;
- Toxic—most commonly alcohol but there are very many hepatotoxic medications (Table 2) and toxins;
- Endocrine e.g., metabolic dysfunction-associated steatotic liver disease;
- Metabolic e.g., haemochromotosis or Wilson;
- Traumatic/surgical;
- Neoplastic;
- Pulmonary; very large pulmonary emboli, and other pathologies, can cause right heart strain sufficient to impact hepatic function with congestion, hyperbilirubinaemia being an adverse prognostic marker in pulmonary artery hypertension (50).
Decreased excretion/post-hepatic
Biliary obstruction leads to a rise in both conjugated and unconjugated bilirubin, but predominantly the former. As the bilirubin does not reach the gut then stool becomes pale and conjugated bilirubin in the urine can make it very dark (Figure 2B).
In many post-hepatic causes of hyperbilirubinaemia, ALP will be elevated proportionally higher in relation to the other liver enzymes (degree > upper limit of normal) and, in combination with an elevated GGT activity, confirms a hepatic cause (49). An AST:ALT ratio can also be useful in determining the cause of hyperbilirubinaemia, whereby an AST:ALT ratio of <1.5 suggests an intrahepatic cause and a ratio of >1.5 suggests an extrahepatic cause (51). Biliary obstruction can lead to hypercholesterolaemia due to lipoprotein-X, therefore hypercholesterolaemia is supportive of cholestasis (52).
Causes of obstructed bile flow include:
- Physical blockage from tumours, stones, strictures, cysts;
- Hepatocellular damage (oedema crushes the ducts);
- Drugs (see Table 2);
- Metabolic and endocrine disorders e.g., hypothyroidism (53);
- Autoimmune e.g., primary sclerosing cholangitis;
- Inborn errors of metabolism (54,55).
Pregnancy and paediatrics
Pregnancy is associated with multiple metabolic changes; perhaps most notable is significant haemodilution. However, in an uncomplicated pregnancy the bilirubin is expected to remain within normal limits (56). There are several relevant conditions associated with pregnancy that can cause an alteration in bilirubin concentration:
- Extreme cases of hyperemesis gravidarum; approximately 50–60% of patients have an elevation in AST and ALT but jaundice only occurs in those who have reached severe dehydration and starvation;
- Syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP); cause remains unclear but associated with pre-eclampsia and fatty acid oxidation defects (57);
- Acute fatty liver of pregnancy (AFLP); diagnosed by ultrasound;
- Intrahepatic cholestasis; most common pregnancy-related liver disease and can result in meconium-stained fluid, spontaneous preterm birth and fetal asphyxia.
Paediatrics
The focus of this article is hyperbilirubinaemia in adults however a few notable paediatric conditions will be discussed. Fetuses do not express UDP-GT and so cannot conjugate bilirubin until 3 months old therefore neonates are prone to jaundice (unconjugated, from day 1–15 of life) (58). By 2 years of age the liver is at full adult capacity and is daily only using 1% of its full metabolic potential. However genetic mutations in UDP-GT can lead to partial or complete loss of function seen in the inherited hyperbilirubinaemias (see Table 3) and Gilbert disease is a common cause of elevated bilirubin in adults with up to a 10% prevalence in Western Europe (NICE). Very high bilirubin (>300 µmol/L) can damage the brain so phototherapy, and occasionally phenobarbital, is used to treat neonates at risk of kernicterus. Lucey-Driscoll syndrome is a controversial entity possibly related to the presence of a transient inhibitor of UDP-GT at birth (59,62,63). If conjugated bilirubin is elevated in neonates and present within the first 24 hours or continues after 2 weeks, then it is pathological rather than physiological. A full discussion of the diagnosis of hepatitic patterns, which will include diseases that present in childhood e.g., Wilson or α1 antitrypsin deficiency, will be covered in a sister article to this series on the investigation of raised AST/ALT. Breast feeding jaundice is a jaundice in a newborn lasting longer than the neonatal period. The precise mechanism by which breast feeding jaundice occurs remains unclear however it is believed to be due to a combination of possible inhibitory components present in breast milk or polymorphisms in the newborn’s genes that impact on bilirubin metabolism (64). Treatment is not usually indicated (65).
Table 3
| Name of syndrome | Gilbert syndrome | Crigler Najjar syndrome | Dubin Johnson syndrome | Rotor syndrome | Neonatal jaundice |
|---|---|---|---|---|---|
| Inheritance | Autosomal recessive | Autosomal recessive | Autosomal recessive | Autosomal recessive | – |
| Gene involved | UGT1A1 | UGT1A1 | ABCC2 | SLCO1B1 and SLCO1B3 | GSTM1 |
| Protein affected by mutations | Uridine disphosphate-glucuronosyltransferase-1 | Uridine disphosphate-glucuronosyltransferase-1 | MRP2/canalicular multispecific organic anion transporter | Organic anion transporting polypeptide 1B1 and 1B3 | Glutathione s transferase |
| Standard biochemistry | 50% or less enzyme function, mild symptoms, unconjugated bilirubin between normal to 100 µmol/L | Type 1 is complete loss of function, unconjugated bilirubin 300–600 µmol/L); type 2 10% function (100–400 µmol/L) | Normal liver enzymes, prolonged prothrombin time, mild conjugated bilirubinaemia (35–100 µmol/L) | Bilirubin, predominantly conjugated, mildly elevated (35–100 µmol/L) | Unconjugated bilirubin |
| Additional biochemical tests | Normal (75% coproporphyrin III) | Normal (75% coproporphyrin III) | Normal total coproporphyrin (80% coproporphyrin I) | 3–5 fold increase in coproporphyrin (65% coproporphyrin I) | Normal (75% coproporphyrin III) |
MRP2, multidrug resistance protein 2.
Conclusions
This article presents a laboratory approach to help define and distinguish causes for a raised bilirubin in human serum. It is hoped the algorithm will be useful in daily clinical practice to help guide investigations and elucidate the reasons for the biochemical derangement but it is not a substitute for clinical assessment and expert guidelines. The strengths of this review are that it is based on established practice and aimed at being applicable for most health care settings, focussed on common presentations, and accessible for all users. The limitations are that a critical appraisal or systematic review were outside the scope of the article and also that some special patient groups may not be well represented e.g., neonatal. The cost and clinical efficiency of such a schema is therefore unknown but by providing a suggested approach this will allow the pathway to be validated and the performance quantified if implemented. At the least we hope to provide a framework for clinicians to consider an approach to further diagnostics when facing a clinical conundrum related to abnormal bilirubin results.
Acknowledgments
We would like to thank Professor Rousseau Gama (BSc, MB ChB, MSc, MD, EuSpLm, CSci, CCHEM, FRSC, FRCP, FRCPath) from Black Country Pathology Services, The Royal Wolverhampton NHS Trust, Wolverhampton, UK, Department of Laboratory and Metabolic Medicine, School of Medicine and Clinical Practice, Wolverhampton University, UK, for inspiring and inviting us to prepare this paper. All figures were created with BioRender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Investigative Algorithms in Laboratory Medicine II: Focus on Bone and Liver”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-24/rc
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-24/coif). The series “Investigative Algorithms in Laboratory Medicine II: Focus on Bone and Liver” was commissioned by the editorial office without any funding or sponsorship. K.E.S. served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- London IM, West R, Shemin D, et al. On the origin of bile pigment in normal man. J Biol Chem 1950;184:351-8. [Crossref] [PubMed]
- Tenhunen R, Ross ME, Marver HS, et al. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry 1970;9:298-303. [Crossref] [PubMed]
- Carragher F. Paediatric clinical biochemistry. In: Marshall WJ, Lapsley M, Day AP, et al., editors. Clinical Biochemistry: Metabolic and Clinical Aspects (Third Edition). Churchill Livingstone, 2014:484-96.
- Bosma PJ, Seppen J, Goldhoorn B, et al. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem 1994;269:17960-4. [Crossref] [PubMed]
- Gatmaitan ZC, Nies AT, Arias IM. Regulation and translocation of ATP-dependent apical membrane proteins in rat liver. Am J Physiol 1997;272:G1041-9. [PubMed]
- Watson CJ. The urobilinoids: milestones in their history and some recent developments. In: Berk PD, Berlin NI, editors. Bile Pigments: Chemistry and Physiology. Washington, DC: US Government Printing Office, 1977 pp. 469-82.
- Fargo MV, Grogan SP, Saguil A. Evaluation of Jaundice in Adults. Am Fam Physician 2017;95:164-8. [PubMed]
- Mustafa MG, Cowger ML, King TE. Effects of bilirubin on mitochondrial reactions. J Biol Chem 1969;244:6403-14. [Crossref] [PubMed]
- Sano K, Nakamura H, Matsuo T. Mode of inhibitory action of bilirubin on protein kinase C. Pediatr Res 1985;19:587-90. [Crossref] [PubMed]
- Schiff D, Chan G, Poznansky MJ. Bilirubin toxicity in neural cell lines N115 and NBR10A. Pediatr Res 1985;19:908-11. [Crossref] [PubMed]
- Hansen TWR. Core Concepts: Bilirubin Metabolism. Neo Reviews 2010;11:e316-22. [Crossref]
- Brodersen R. Aqueous solubility, albumin binding, and tissue distribution of bilirubin. In: Ostrow JD, editor. Bile Pigments and Jaundice; Molecular, Metabolic and Medical Aspects. 1986. Marcel Dekker, New York, NY. 157-181.
- Fevery J. Bilirubin in clinical practice: a review. Liver Int 2008;28:592-605. [Crossref] [PubMed]
- McDonagh AF. Thermal and photochemical reactions of bilirubin IX. Ann NY Acad Sci 1975;244:553-69. [Crossref] [PubMed]
- Itoh S, Onishi S. Kinetic study of the photochemical changes of(ZZ)-bilirubin IX bound to human serum albumin. Demonstration of (EZ)-bilirubin IX as an intermediate in photochemical changes from(ZZ)-bilirubin IX to (EZ)-cyclobilirubin IX. Biochem J 1985;226:251-8. [Crossref] [PubMed]
- Sofronescu AG, Loebs T, Zhu Y. Effects of temperature and light on the stability of bilirubin in plasma samples. Clin Chim Acta 2012;413:463-6. [Crossref] [PubMed]
- Heirwegh KP, Fevery J, Blanckaert N. Chromatographic analysis and structure determination of biliverdins and bilirubins. J Chromatogr 1989;496:1-26. [Crossref] [PubMed]
- Dolscheid-Pommerich RC, Klarmann-Schulz U, Conrad R, et al. Evaluation of the appropriate time period between sampling and analyzing for automated urinalysis. Biochem Med (Zagreb) 2016;26:82-9. [Crossref] [PubMed]
- Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem 1973;119:481-90. [Crossref]
- Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry, 3rd ed. Philadelphia, PA: WB Saunders; 1999:1136-7.
- Walters MI, Gerarde HW. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchemical Journal 1970;15:231-43. [Crossref]
- Winsten S, Cehelyk B. A rapid micro diazo technique for measuring total bilirubin. Clin Chim Acta 1969;25:441-6. [Crossref] [PubMed]
- Association for Chemical Biochemistry and Laboratory Medicine. Analyte Monographs: Bilirubin [Internet]. [cited 2023 Sep 1]. Available online: https://www.acb.org.uk/
- Binder L, Smith D, Kupka T, et al. Failure of prediction of liver function test abnormalities with the urine urobilinogen and urine bilirubin assays. Arch Pathol Lab Med 1989;113:73-6. [PubMed]
- Melville A, Thomas SDC. Paraprotein interference of automated total bilirubin measurement. Pathology 2022;54:365-7. [Crossref] [PubMed]
- Blanckaert N, Kabra PM, Farina FA, et al. Measurement of bilirubin and its monoconjugates and diconjugates in human serum by alkaline methanolysis and high-performance liquid chromatography. J Lab Clin Med 1980;96:198-212. [PubMed]
- Jalan R, Hayes PC. Review article: quantitative tests of liver function. Aliment Pharmacol Ther 1995;9:263-70. [Crossref] [PubMed]
- Wexner Medical. Point of Care Multistix [Internet]. [cited 2023 Apr 30]. Available online: https://wexnermedical.osu.edu/-/media/files/wexnermedical/healthcare-professionals/clinical-labs/forms-policies-procedures/point-of-care/clinitek/multistix-package-insert.pdf?%20la=en&hash=66CBDB2DBCA88640757CECD8F58AD8D70E666DB8
- Foley KF, Wasserman J. Are unexpected positive dipstick urine bilirubin results clinically significant? A retrospective review. Lab Med 2014;45:59-61. [Crossref] [PubMed]
- Wang Q, Zhang T, Lin Y, et al. Accuracy and Reliability of Whole Blood Bilirubin Measurements Using a Roche Blood Gas Analyzer for Neonatal Hyperbilirubinemia Screening and Risk Stratification. Front Pediatr 2022;10:910566. [Crossref] [PubMed]
- Huang Y, Dean R, Dubbelman Y, et al. Neonatal hemoglobin affects the accuracy of whole blood bilirubin measurement on GEM Premier 4000 blood gas analyzers. Pract Lab Med 2021;25:e00231. [Crossref] [PubMed]
- McCudden CR, Fleming K, Warr M. Robustness of the Reichert Unistat Bilirubinometer for analysis of hemolyzed samples from neonates. Clin Biochem 2017;50:238-40. [Crossref] [PubMed]
- Chung JO, Park SY, Chung DJ, et al. Relationship between anemia, serum bilirubin concentrations, and diabetic retinopathy in individuals with type 2 diabetes. Medicine (Baltimore) 2019;98:e17693. [Crossref] [PubMed]
- Jouppila R, Larva L, Jouppila P, et al. Effect of segmental epidural analgesia on neonatal serum bilirubin concentration and incidence of neonatal hyperbilirubinemia. Acta Obstet Gynecol Scand 1983;62:179-82. [Crossref] [PubMed]
- Testa R, Bardellini E, Borzone S, et al. Caffeine clearance in subjects with constitutional unconjugated hyperbilirubinemia. Ital J Gastroenterol 1995;27:129-32. [PubMed]
- Kuno T, Togawa H, Mizutani T. Induction of human UGT1A1 by a complex of dexamethasone-GR dependent on proximal site and independent of PBREM. Mol Biol Rep 2008;35:361-7. [Crossref] [PubMed]
- Eghbalian F, Hasanpour-Dehkordi A, Raeisi R. The Effects of Clofibrate on Neonatal Jaundice: A Systematic Review. Int J Prev Med 2022;13:3. [Crossref] [PubMed]
- Meisel P, Amon I, Hüller H, et al. Effect of theophylline on the riboflavin-sensitized photodegradation of bilirubin in vitro. Biol Neonate 1980;38:30-5. [Crossref] [PubMed]
- Blaschke TF, Berk PD, Rodkey FL, et al. Drugs and the liver. I. Effects of glutethimide and phenobarbital on hepatic bilirubin clearance, plasma bilirubin turnover and carbon monoxide production in man. Biochem Pharmacol 1974;23:2795-806. [Crossref] [PubMed]
- Chattopadhyay N, Kanacher T, Casjens M, et al. CYP3A4-mediated effects of rifampicin on the pharmacokinetics of vilaprisan and its UGT1A1-mediated effects on bilirubin glucuronidation in humans. Br J Clin Pharmacol 2018;84:2857-66. [Crossref] [PubMed]
- Van Vlierberghe H, Langlois M, Delanghe J. Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta 2004;345:35-42. [Crossref] [PubMed]
- Hill QA, Stamps R, Massey E, et al. Guidelines on the management of drug-induced immune and secondary autoimmune, haemolytic anaemia. Br J Haematol 2017;177:208-20. [Crossref] [PubMed]
- Hill QA, Stamps R, Massey E, et al. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol 2017;176:395-411. [Crossref] [PubMed]
- Strobel E. Haemolytic transfusion reactions. Transfus Med Hemother 2008;35:346-53. [Crossref] [PubMed]
- Rahmat J, Gelfand RL, Gelfand MC, et al. Captopril-associated cholestatic jaundice. Ann Intern Med 1985;102:56-8. [Crossref] [PubMed]
- Poventud-Fuentes I, Garnett E, Despotovic J, et al. Interference of eltrombopag with bilirubin measurements on the Vitros 5600 analyzer. J Clin Lab Anal 2021;35:e23796. [Crossref] [PubMed]
- Visentin M, Stieger B, Merz M, et al. Octreotide inhibits the bilirubin carriers organic anion transporting polypeptides 1B1 and 1B3 and the multidrug resistance-associated protein 2. J Pharmacol Exp Ther 2015;355:145-51. [Crossref] [PubMed]
- Gibbs PE, Tudor C, Maines MD. Biliverdin reductase: more than a namesake - the reductase, its Peptide fragments, and biliverdin regulate activity of the three classes of protein kinase C. Front Pharmacol 2012;3:31. [Crossref] [PubMed]
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med 2010;77:195-204. [Crossref] [PubMed]
- Takeda Y, Takeda Y, Tomimoto S, et al. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med 2010;10:22. [Crossref] [PubMed]
- Hall P, Cash J. What is the real function of the liver 'function' tests?. Ulster Med J 2012;81:30-6. [PubMed]
- Seidel D, Büff HU, Fauser U, et al. On the metabolism of lipoprotein-X (LP-X). Clin Chim Acta 1976;66:195-207. [Crossref] [PubMed]
- Van Steenbergen W, Fevery J, De Groote J. Thyroid hormones and the hepatic handling of bilirubin. II. Effects of hypothyroidism and hyperthyroidism on the apparent maximal biliary secretion of bilirubin in the Wistar rat. J Hepatol 1988;7:229-38. [Crossref] [PubMed]
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007;262:254-62. [Crossref] [PubMed]
- Heubi JE, Setchell KDR, Bove KE. Inborn Errors of Bile Acid Metabolism. Clin Liver Dis 2018;22:671-87. [Crossref] [PubMed]
- Brady CW. Liver Disease in Pregnancy: What's New. Hepatol Commun 2020;4:145-56. [Crossref] [PubMed]
- Preece MA, Green A. Pregnancy and inherited metabolic disorders: maternal and fetal complications. Ann Clin Biochem 2002;39:444-55. [Crossref] [PubMed]
- Burchell B, Coughtrie M, Jackson M, et al. Development of human liver UDP-glucuronosyltransferases. Dev Pharmacol Ther 1989;13:70-7. [Crossref] [PubMed]
- Memon N, Weinberger BI, Hegyi T, et al. Inherited disorders of bilirubin clearance. Pediatr Res 2016;79:378-86. [Crossref] [PubMed]
- Muslu N, Dogruer ZN, Eskandari G, et al. Are glutathione S-transferase gene polymorphisms linked to neonatal jaundice? Eur J Pediatr 2008;167:57-61. [Crossref] [PubMed]
- Abdel Ghany EA, Hussain NF, Botros SK. Glutathione S-transferase gene polymorphisms in neonatal hyperbilirubinemia. J Investig Med 2012;60:18-22. [Crossref] [PubMed]
- Arias IM, Wolfson S, Lucey JF, et al. Transient familial neonatal hyperbilirubinemia. J Clin Invest 1965;44:1442-50. [Crossref] [PubMed]
- Kaabneh MA, Salama GS, Shakkoury AG, et al. Phenobarbital and Phototherapy Combination Enhances Decline of Total Serum Bilirubin and May Decrease the Need for Blood Exchange Transfusion in Newborns with Isoimmune Hemolytic Disease. Clin Med Insights Pediatr 2015;9:67-72. [Crossref] [PubMed]
- Preer GL, Philipp BL. Understanding and managing breast milk jaundice. Arch Dis Child Fetal Neonatal Ed 2011;96:F461-6. [Crossref] [PubMed]
- Bratton S, Cantu RM, Stern M. Breast Milk Jaundice. [Updated 2023 Jan 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
Cite this article as: Verran C, Shipman AR, Shipman KE. Investigative algorithms for disorders affecting plasma bilirubin: a narrative review. J Lab Precis Med 2024;9:6.


