
Investigative algorithms for disorders affecting plasma lactate dehydrogenase: a narrative review
Introduction
The measurement of plasma lactate dehydrogenase (LDH) initially became widespread due to its role in detecting acute myocardial infarction (1). However, LDH gained popularity in a wide range of medical and surgical specialties due to its almost ubiquitous tissue location (2). Plasma LDH activity therefore may be performed as part of screens when a clinician is faced with an unwell patient and is also part of various staging and monitoring algorithms (3). As a wide range of normal tissues and tumors can produce LDH, an isolated elevated LDH is not diagnostic of any single condition, therefore clinical clues and other investigations are required to elucidate the cause of an LDH elevation.
The following paper will discuss how to approach deviations of LDH concentrations from the normal range and provide a systematic laboratory algorithm, which can be used to help the clinician account for plasma LDH activity elevation when struggling to find the diagnosis. We will not cover the investigation of LDH in bodily fluids. The algorithms are designed for all healthcare professionals irrespective of their expertise, or lack of expertise, with diagnostics and are meant to provide an up-to-date suggestion to guide management steps that can be validated in the future in a variety of healthcare settings and patient populations. We present this article in accordance with the Narrative Review reporting checklist (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-65/rc).
Methods
The limited narrative literature review was created by searching Medline, Google Scholar, OMIM and seminal texts over the period April 2023 to September 2023. The diagnostic algorithms were then created based on the literature review by the authors. The language was restricted to English and excluded if non-human studies. For further information please see Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | April to September 2023 |
| Databases and other sources searched | Medline, Google Scholar, OMIM |
| Search terms used | LDH, lactate dehydrogenase, Low, High, Raised, Tissue, Activity, Drugs, Investigations, Algorithms, Guidelines, Diagnosis, Causes, Aetiology, Human |
| Timeframe | From database inception to September 2023 |
| Inclusion and exclusion criteria | All papers and reviews were included but restricted to English. Animal data were excluded |
| Selection process | A.R.S. and S.B. conducted initial search, with refinement by all other authors to obtain consensus and agreement |
| Any additional considerations, if applicable | Seminal texts were also searched and the references of important articles and texts were obtained and checked for relevance |
OMIM, Online Mendelian Inheritance in Man.
What is LDH?
LDH is a universally expressed enzyme across all cells and conserved between organisms. LDH concentrations are highest in high energy consuming organs such as muscles, liver, kidneys, lungs, heart, and blood cells (4). LDH is involved in the anaerobic metabolic pathway (Figure 1) (3,4). LDH reversibly converts pyruvate to lactate, and hydrogenated nicotinamide adenine dinucleotide (NADH) to nicotinamide adenine dinucleotide (NAD+), and generates adenosine triphosphate (ATP) when oxygen supplies dwindle (3,4). At the molecular level, LDH is a tetramer comprising two common subunits, A and B, with subunit C predominating in sperm and testes. Chromosomes 11 and 12 host the genes encoding these subunits and various combinations—homo- or heterotetrameric—yield a diverse assortment of five LDH isoforms, called LDH1 to LDH5 (3,4). Although all these isoenzymes facilitate the same catalytic reaction, their structural composition, substrate affinity, temperature responsiveness, and tissue-specific distributions diverge markedly (3,4). Distinguishing between them however is not a test commonly available to most clinicians.
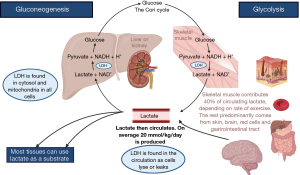
How is LDH measured?
LDH is quantified by measurement of the enzymatic activity of the specimen rather than concentration of the enzyme. When measuring the enzyme activity one can either detect the formation of lactate from pyruvate, which is the internationally recommended reference method described as “forward” or “lactate pyruvate (LP)”, or vice versa [“backward” or “pyruvate lactate (PL)”] (5,6). This has allowed reference material to be produced and therefore an ability to harmonize assays internationally making reference intervals potentially comparable (7,8). However, there is a variety of methods used internationally (6). In addition to the direction of the reaction, different reagents and buffers can be used resulting in very discrepant results between assay manufacturers preventing harmonization (9).
LDH isoenzymes can be separated and quantified by electrophoresis but other biomarkers have replaced this test and it is no longer routinely performed (10). Electrophoresis may also detect ‘macroLDH’, a spurious cause of increased activity (11). In the “macro” condition antibody or cell wall components bind LDH preventing clearance and hence causing accumulation that is not related to an increased rate of release from the tissue of origin (12).
Although antibody binding can cause increased accumulation of LDH, and hence an increase in serum activity which can be spuriously attributed to tissue damage, it may also inhibit enzymatic activity analytically causing a spuriously low measured level even in the face of an increased serum concentration (13,14). Other causes of spurious results include in vitro haemolysis releasing LDH into the tube and elevating LDH activity in the specimen (15). Spuriously raised LDH activities have also been reported secondary to triamterene (16). Potentially spuriously low LDH activities have been reported secondary to hyperlipidaemia, aspirin and vitamin C (17-19). Small elevations in activity of LDH in lymphoma may not always be indicative for change in tumour burden (20).
Causes of raised LDH activity
Elevated LDH activity arises due to multiple aetiological factors affecting almost every organ system (2). Commoner organ system causes of LDH elevation are either because the tissue has greater mass, more LDH expression or because the diseases are the most common. For example, in one study of 500 people with elevated LDH activity the pathology identified, in descending order of frequency, was (21):
- Cardiorespiratory diseases;
- Malignancy;
- Fracture;
- Central nervous system disease;
- Infection/inflammation;
- Hepatic cirrhosis and/or alcoholism;
- Trauma without fracture;
- Infectious mononucleosis;
- Hypothyroidism;
- Uraemia;
- Necrosis;
- Pseudo-mononucleosis;
- Viraemia;
- Intestinal obstruction;
- Idiopathic in 3%.
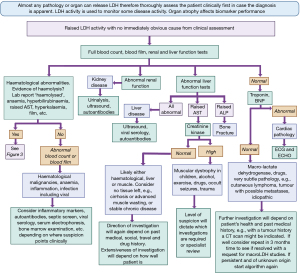
One group tried to see if an elevated LDH activity on admission to hospital could provide any diagnostic or prognostic advantage (22). The cases were defined as those at hospital admission with LDH >800 IU/L [and aspartate transaminase/alanine transaminase (AST/ALT) <60 U/L] and the control group was those admitted with LDH <800 IU/L (and AST/ALT <60 U/L). Diagnoses were similar between the groups, the main notable exceptions were that infections (predominantly pulmonary), solid tumors, liver metastases, and haematological malignancies were more frequent in the case group and cardiovascular disease more common in the controls. With 158 patients in the case group and 188 in the control, and 14 main diagnoses driving admission, power was limited but confirms the fact that LDH remains a non-specific marker and other tests are required to reach a diagnosis. Therefore, whilst we present an algorithm (Figure 2) to help diagnose the cause of an elevated LDH if one is uncertain after clinical assessment, it is important to remember that LDH in general is perhaps better employed as a marker of disease activity, rather than a specific diagnostic tool.
Connective tissue
Adipocytes contain LDH however obesity alone does not seem to cause an elevation in circulating LDH (23). In conditions of underweight patients, elevations to LDH are due to a multitude of causes, e.g., in cancer cachexia the tumor, tissue breaks down and drugs commonly elevate LDH (24). In anorexia nervosa elevated LDH may be due to skeletal muscle breakdown, mediated by low T3 (25). Elevated LDH was also shown by other groups in anorexia nervosa patients (compared to normal controls) but the LDH was also elevated, perhaps not unexpectedly, in a comparator group of those who were anorectic due to bowel surgery or malignancy (26,27). However, another study demonstrated lower LDH in anorexia nervosa compared to controls (28). Perhaps these contradictory results reflect other tissue damage that can occur in anorexia nervosa meaning low body weight alone is insufficient to cause a disturbance compared to, e.g., muscle breakdown, cardiac stress or liver damage (29).
All five LDH tetramers are expressed in skin but LDH-5 is the most common form (30). Burns elevate LDH and the deeper the burn the higher the LDH as the tissue anaerobically respires secondary to the tissue damage (31). However extensive and common skin diseases, such as psoriasis and eczema, do not seem to cause a clinically significant increase in LDH (32-34) However a correlation with LDH activity and the severity of eczema in children has been noted and higher LDH activity predicting poorer response to treatment in these patients (35,36).
LDH measurement has been recommended in various guidelines for skin malignancies, e.g., cutaneous lymphoma and melanoma (37). Elevated LDH levels have demonstrated a correlation with unfavourable prognosis with cutaneous lymphoma and LDH has also been integrated into the tumor-node-metastasis (TNM) staging system for melanoma (38-40). Insights gleaned from cancer databases demonstrate the consistent elevation of LDH expression in malignancies, thereby accentuating its potential role in the meticulous monitoring of metastatic cutaneous tumours (41). The interpretation of LDH measurements within cutaneous malignancy warrants thoughtful consideration and caution, given the absence of definitive trials to substantiate its specific role (3,41).
LDH is also elevated in connective tissue disease conditions characterized by extensive tissue damage, including dermatomyositis, myositis, and panniculitides. This connection means that LDH levels could potentially be used to track disease activity within these contexts (41). Other more traditional rheumatological disorders such as systemic sclerosis might elevate LDH, correlating with a more aggressive and multiorgan disease (42). However cutaneous only sclerosis or lupus do not seem to be related to an elevated LDH in the literature. A spectrum of rheumatological diseases have not related to LDH elevation in one small case series (43) however there are papers of LDH being related to disease activity of Adult Still disease, rheumatoid arthritis and vasculitis for example (44-49).
Bone is another source of LDH and injuries, particularly fractures, will lead to elevation. In athletes, a raised LDH might point towards stress fractures for example with the correct history (50). Raised LDH is a poor prognostic indicator in hip fracture patients, although whether the LDH levels is related to the degree of bone injury or rather muscle death from ischaemia, haemorrhage, long lie, cardiac stress, etc. is not certain (51). Certainly, an early study showed that operative repair of hip fractures did not cause a significant change in LDH and therefore, whilst the bone fracture and muscle damage can increase LDH serum activity levels, if there is a significant elevation of LDH then the clinician needs to consider if there are other sources, e.g., a cardiac event (52). LDH has also been shown to rise in rare sclerosing bone pathologies (53).
Nervous and muscular systems
Lactate metabolism increases in brain injury and multiple pathologies have been related to elevated LDH in serum including intracerebral haemorrhage, ischaemic or haemorrhagic stroke, post-epileptic seizures (and indeed can be used as a biochemical marker to distinguish an epileptic versus non-epileptic seizure), infectious meningitis and encephalitis, cerebral oedema, encephalopathy related to pre-eclampsia, post brain surgery with complications and traumatic brain injuries (54-63).
Cardiac muscle is another common source of LDH elevation, with LDH once being used as a marker for myocardial infarction before being replaced with troponin T (64,65) and so will be elevated post cardiac arrest, shock and hypoxia. Cardiomyopathies, heart failure, valve disease, QTc prolongation, pulmonary hypertension, arrhythmia such as atrial fibrillation, rheumatic heart disease (66), prosthetic valves (particularly if leaking) and trauma causing intravascular haemolysis and pre-eclampsia can all cause LDH elevation and again LDH is often not used diagnostically but as a marker of disease activity or prognostication (67-74).
It should be noted that LDH is in smooth muscle too (75), so whilst perhaps a rarer cause of serum LDH elevation any damage to smooth muscle can contribute to LDH elevation. Smooth muscle is present in blood vessels, uterus, ureteric tracts to name just a few systems. Smooth muscle pathologies are unusual, but a few examples include:
- Autoimmune diseases, e.g., anti-smooth muscle antibodies often associated with autoimmune hepatitis;
- Tumours, e.g., leiomyomas and leiomyosarcomas, the latter often causing a greater elevation in LDH (76-78).
Skeletal muscle breakdown will release LDH into the serum. Any cause of muscle breakdown will trigger this, e.g.,
- Alcoholic myopathy (79);
- Inflammatory myositis (80);
- Muscular dystrophy (81);
- Strenuous exercise (82);
- Drug induced, e.g., steroids, statins, quinines (83).
As muscle is destroyed there is less tissue to release LDH and so if a disease has already caused significant muscle atrophy but is still active it may not produce high LDH activity (unless for other reasons, e.g., increased cardiac stress due to failing skeletal muscles).
Haematological
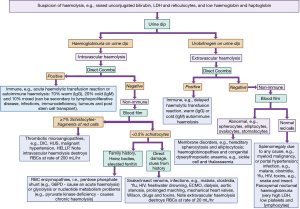
Haemolysis is a common cause of LDH elevation. Figure 3 shows a laboratory diagnostic algorithm approach to haemolysis (from sister article in this series on bilirubin investigation with identical methodology). Severe pernicious anaemia can cause an LDH elevation (84) as can megaloblastic anaemia (85), sickle cell (86,87), Epstein-Barr virus (88), thrombocythaemia (89), haematological malignancy (90), polycythaemia (91), splenic necrosis (92) and paroxysmal nocturnal haemoglobinuria (93). However, a normal LDH does not preclude a haematological malignancy, for example, up to 80% of patients with myeloma will have normal LDH activity (94). The worse the disease the more likely the elevation of LDH and higher LDH is a clue to a worse prognosis. It should be noted that it also possible for inflammatory diseases with high lymphocyte activity to lead to a raised LDH, e.g., T lymphocytes in psoriasis (32) or lymphadenopathy in sarcoidosis (95).
Other viscera
A study demonstrated elevated LDH in 204 newly diagnosed cervical cancer patients but unfortunately, they were compared to healthy controls rather than to those with cervical dysplasia or HPV infections, and whilst statistically significant the median LDH values were 194 U/L [interquartile range (IQR), 65.25 U/L] in patients compared to 180 U/L (IQR, 40.75 U/L) in controls (96). However, another study demonstrated a higher LDH pre-cervical cancer treatment was associated with a worse prognosis (97). Ovarian tumors can elevate LDH, and in children point towards dysgerminomas in particular (98) but LDH is also elevated in benign gynaecological disease (99). Whilst LDH activity will be elevated more in malignant gynae pathology the degree of elevation is not great enough to allow for a confident discrimination from benign disease using LDH activity alone (100).
Renal transplant rejection has also been noted to cause an elevation in LDH (101) and LDH has been noted to increase post dialysis although mechanical trauma might be the reason for this elevation (102). Nephropathies and chronic kidney disease of any cause can also elevate LDH, of course the more severe disease, especially if there is infarction, the greater the elevation (103-109). LDH can be used a poor prognostic marker in acute kidney injury and renal cell carcinoma and also a marker of multiple organ involvement in a particular illness (110,111).
LDH can be used as a prognostic marker for the refractory nature of mycoplasma pneumonia, for example in children a cut off 408 IU/L has an area under the receiver operating characteristic curve (AUROC) of 0.812 (sensitivity of 75% and specificity of 72.2%), i.e., LDH higher than 408 IU/L predicts the pneumonia will be refractory to treatment (112). One group showed that elevated LDH after subarachnoid haemorrhage statistically significantly predicted post operative pneumonia (113) however their LDH values overlapped (pneumonia group 261.26±126.51 U/L vs. no pneumonia group 189.00±69.20 U/L) perhaps reflecting that the brain injury itself plus any seizures, surgery etc. might also be putting the LDH up in this situation.
Pulmonary embolus and infarction will elevate LDH (114) but not of a degree to help distinguish between myocardial and pulmonary infarct in the setting of chest pain and breathlessness (115). LDH elevation does seem to distinguish between children with asthma, compared to controls (116) and there may be a correlation of LDH with prognosis in chronic obstructive pulmonary disease in adults and interstitial lung disease (117,118). LDH elevation is perhaps more closely link to pneumonia compared to other causes of infection in acute hospital admissions but the actual difference between LDH levels does not render it a useful clinical test in this setting (119).
The gastrointestinal tract is another source of LDH in the serum. Pancreatitis, pancreatic necrosis or mesenteric ischaemia cause LDH elevation as does bowel gangrene (120-122). Any liver pathology can elevate LDH including hepatitis, cholestasis, trauma and cirrhosis, and again higher levels are more likely to be related to poor prognosis and mortality in both liver disease and peritonitis (123,124). Stomach pathologies do not seem to elevate LDH, whether benign or malignant (125).
Hypothyroidism can lead to an elevated LDH due to skeletal muscle breakdown and hyperthyroidism to a relative decrease (126,127). LDH is an important enzyme in phaeochromocytoma (128) and has also been shown to be raised in diabetes mellitus and Cushing syndrome (129,130).
Diseases
Tumours, particularly the malignant and metastatic, often have a higher metabolism so take up more glucose [hence the rationale of positron emission tomography (PET) imaging] and produce more lactate, via LDH activity. The higher the LDH the more active the cells metabolically but also the LDH activity seems to suppress immune surveillance and therefore helps the tumour survive (131). This review will not tackle the prognostic indication that LDH might provide with cancer monitoring but it is widely used in the oncology setting, partly as a marker of tumour burden and monitoring changes to the levels indicating treatment success or relapse. LDH activity has been shown to be elevated in many tumours, particularly metastatic breast, colorectal, germinal testicular, gastric, hepatoma, lung, ovarian, pancreatic, melanoma, prostatic, renal, sarcoma, seminoma and thyroid (132,133). Chemotherapy can lead to LDH rises initially, as expected, due to tumor breakdown (134).
Infection can cause LDH elevation, e.g., human immunodeficiency virus (HIV), usually in acquired immunodeficiency syndrome (AIDS)-defining infections or coronavirus disease 2019 (COVID-19) (135,136). There is an unusual LDH elevating virus, although this seems to be specific to mice (137). Malaria is a cause of LDH elevation, partly due to its effects on red cells (138). Again, the worse the infection the more likely one will detect an LDH elevation.
Low LDH
There are multiple different genetic conditions described as causing an LDH deficiency. In most, there is no disease and instead what happens is that there is an unusually low LDH activity than expected on laboratory testing (Table 2) (139,140). However, the lack of LDH, as could be expected, can impair the person’s ability to perform physical activity and progress in labour (141). Clinically low LDH is not something that is ever a cause for clinical concern.
Table 2
| Genotype | Title | Phenotype |
|---|---|---|
| LDHA, autosomal recessive, chromosome 11 | Glycogen storage disease XI or lactate dehydrogenase A deficiency | Exertional myoglobinuria, easy fatigue, exercise-induced elevation of lactate, pyruvate, creatine kinase with myoglobinuria. Can develop renal failure. Skin rash—non-pruritic erythemato-squamous patches |
| LDHB, chromosome 12 | Lactate dehydrogenase B deficiency | Does not seem to cause a disease, just a low reading of lactate dehydrogenase activity than normal values |
| LDHC, chromosome 11 | Lactate dehydrogenase C deficiency (testicular form) | Impaired sperm motility |
| LDHD, chromosome 16, autosomal recessive | D-lactic aciduria with gout | Elevated D-lactate in plasma and urine, elevated serum uric acid, low urinary uric acid levels, reduced renal clearance of uric acid and gouty arthropathy |
LDHA, lactate dehydrogenase A gene; LDHB, lactate dehydrogenase B gene; LDHC, lactate dehydrogenase C gene; LDHD, lactate dehydrogenase D gene.
Special circumstances
Pregnancy
A normal pregnancy does not increase LDH and if LDH activity increases then one needs to consider disease like pre-eclampsia, pregnancy induced hypertension and cholestasis of pregnancy (142-144).
Childhood
LDH activity is higher in children, dropping as they age and reaching adult levels by the age of 22 (145). An idea of the range in paediatric population is therefore helpful to guide if a child needs further investigation or not (Table 3) (146).
Table 3
| Age | Lower limit | Upper limit | Sample size | Lower confidence limit | Upper confidence limit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |||||
| 0–14 days | 309 | 309 | 1,222 | 1,222 | 197 | 197 | 267, 360 | 267, 360 | 1,116, 1,257 | 1,116, 1,257 | ||||
| 15 days to <1 year | 163 | 163 | 452 | 452 | 145 | 145 | 94, 173 | 94, 173 | 428, 483 | 428, 483 | ||||
| 1 to <10 years | 192 | 192 | 321 | 321 | 370 | 370 | 189, 199 | 189, 199 | 314, 333 | 314, 333 | ||||
| 10 to <15 years | 157 | 170 | 272 | 283 | 141 | 141 | 130, 162 | 138, 175 | 258, 308 | 277, 286 | ||||
| 15 to 19 years | 130 | 130 | 250 | 250 | 227 | 227 | 124, 142 | 124, 142 | 239, 257 | 239, 257 | ||||
Conclusions
Elevated LDH activity is a common finding and is not diagnostic for any single disease process in isolation. The most common causes included cardiorespiratory diseases, any malignancy, fracture or trauma, infection, inflammation and any cause of hepatitis. Low LDH activity is rare and genetic causes might be considered if testing is available. If a thorough clinical assessment does not reveal a cause of LDH elevation then the included algorithm is presented to help support a systematic approach to determine possible reasons. There may be no single cause, or rather multiple tissue sources of the LDH, and in the setting of a well person the result may be spurious. Specialist knowledge, experience and local guidelines all remain vital in elucidating the cause. It is recommended that LDH is not measured unless the clinician has a very specific question in mind due to the non-specificity of serum LDH activity.
Acknowledgments
All figures were created by the authors with BioRender.com and are original.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Investigative Algorithms in Laboratory Medicine II: Focus on Bone and Liver”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-65/rc
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-65/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-65/coif). The series “Investigative Algorithms in Laboratory Medicine II: Focus on Bone and Liver” was commissioned by the editorial office without any funding or sponsorship. K.E.S. served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of the Journal of Laboratory and Precision Medicine from September 2022 to August 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vasudevan G, Mercer DW, Varat MA. Lactic dehydrogenase isoenzyme determination in the diagnosis of acute myocardial infarction. Circulation 1978;57:1055-7. [Crossref] [PubMed]
- Markert CL. Lactate dehydrogenase. Biochemistry and function of lactate dehydrogenase. Cell Biochem Funct 1984;2:131-4. [Crossref] [PubMed]
- Forkasiewicz A, Dorociak M, Stach K, et al. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol Biol Lett 2020;25:35. [Crossref] [PubMed]
- Wu Y, Lu C, Pan N, et al. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Sci Rep 2021;11:12997. [Crossref] [PubMed]
- Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Part 3. Reference procedure for the measurement of catalytic concentration of lactate dehydrogenase. Clin Chem Lab Med 2002;40:643-8. [PubMed]
- Cattozzo G, Guerra E, Ceriotti F, et al. Commutable calibrator with value assigned by the IFCC reference procedure to harmonize serum lactate dehydrogenase activity results measured by 2 different methods. Clin Chem 2008;54:1349-55. [Crossref] [PubMed]
- Siekmann L, Bonora R, Burtis CA, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 7. Certification of four reference materials for the determination of enzymatic activity of gamma-glutamyltransferase, lactate dehydrogenase, alanine aminotransferase and creatine kinase accord. Clin Chem Lab Med 2002;40:739-45. [PubMed]
- Schumann G, Klauke R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta 2003;327:69-79. [Crossref] [PubMed]
- Jansen R, Schumann G, Baadenhuijsen H, et al. Trueness verification and traceability assessment of results from commercial systems for measurement of six enzyme activities in serum: an international study in the EC4 framework of the Calibration 2000 project. Clin Chim Acta 2006;368:160-7. [Crossref] [PubMed]
- Louderback AL, Shanbrom E. Lactic dehydrogenase isoenzyme electrophoresis. JAMA 1968;205:294-5. [Crossref] [PubMed]
- Gemma R, Suzuki Y, Tanaka I, et al. Lactate dehydrogenase (LDH)-linked immunoglobulin in a patient with Graves' disease treated with methimazole. Intern Med 1992;31:377-9. [Crossref] [PubMed]
- Sturk A, Sanders GT. Macro enzymes: prevalence, composition, detection and clinical relevance. J Clin Chem Clin Biochem 1990;28:65-81. [PubMed]
- Fujita K, Takeya C, Saito T, et al. Macro lactate dehydrogenase: an LDH-immunoglobulin M complex that inhibits lactate dehydrogenase activity in a patient's serum. Clin Chim Acta 1984;140:183-95. [Crossref] [PubMed]
- Hirano T, Matsuzaki H, Miura M, et al. An immunoglobulin G inhibiting lactate dehydrogenase activity. Clin Chim Acta 1986;159:17-25. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Montagnana M, et al. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med 2006;44:311-6. [Crossref] [PubMed]
- Chainuvati T, Harinasuta U, Zimmerman HJ. Spurious elevation of apparent lactate dehydrogenase activity caused by triamterene. Clin Chem 1973;19:1202-4. [Crossref] [PubMed]
- Boice J, Prodanovich M, Howell J, et al. Spurious Decrease in Lactate Dehydrogenase Due to Hyperlipidemia. Lab Med 1978;9:28. [Crossref]
- Lee SH, Pekas EJ, Lee S, et al. The Impact of Aspirin Intake on Lactate Dehydrogenase, Arterial Stiffness, and Oxidative Stress During High-Intensity Exercise: A Pilot Study. J Hum Kinet 2020;72:101-13. [Crossref] [PubMed]
- Taghiyar M, Darvishi L, Askari G, et al. The effect of vitamin C and e supplementation on muscle damage and oxidative stress in female athletes: a clinical trial. Int J Prev Med 2013;4:S16-23. [PubMed]
- William BM, Bongu NR, Bast M, et al. The utility of lactate dehydrogenase in the follow up of patients with diffuse large B-cell lymphoma. Rev Bras Hematol Hemoter 2013;35:189-91. [Crossref] [PubMed]
- Jacobs DS, Robinson RA, Clark GM, et al. Clinical significance of the isomorphic pattern of the isoenzymes of serum lactate dehydrogenase. Ann Clin Lab Sci 1977;7:411-21. [PubMed]
- Erez A, Shental O, Tchebiner JZ, et al. Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr Med Assoc J 2014;16:439-43. [PubMed]
- Johansen MJ, Gade J, Stender S, et al. The Effect of Overweight and Obesity on Liver Biochemical Markers in Children and Adolescents. J Clin Endocrinol Metab 2020;105:dgz010. [Crossref] [PubMed]
- McGovern J, Dolan RD, Simmons CPL, et al. Lactate dehydrogenase: relationship with the diagnostic GLIM criterion for cachexia in patients with advanced cancer. Br J Cancer 2023;128:760-5. [Crossref] [PubMed]
- Kumano H, Kuboki T, Tawara R, et al. Interrelationship between serum muscle enzymes and a low T3 in anorexia nervosa. Endocrinol Jpn 1990;37:583-9. [Crossref] [PubMed]
- Umeki S. Biochemical abnormalities of the serum in anorexia nervosa. J Nerv Ment Dis 1988;176:503-6. [Crossref] [PubMed]
- Montagnese C, Scalfi L, Signorini A, et al. Cholinesterase and other serum liver enzymes in underweight outpatients with eating disorders. Int J Eat Disord 2007;40:746-50. [Crossref] [PubMed]
- Nova E, Lopez-Vidriero I, Varela P, et al. Evolution of serum biochemical indicators in anorexia nervosa patients: a 1-year follow-up study. J Hum Nutr Diet 2008;21:23-30. [Crossref] [PubMed]
- Di Pascoli L, Lion A, Milazzo D, et al. Acute liver damage in anorexia nervosa. Int J Eat Disord 2004;36:114-7. [Crossref] [PubMed]
- Bauhammer I, Sacha M, Haltner E. Validation and stability analysis of a modified lactate dehydrogenase (LDH) test method to be employed for an in vitro viable skin model. Heliyon 2019;5:e01618. [Crossref] [PubMed]
- Cuddihy J, Wu G, Ho L, et al. Lactate dehydrogenase activity staining demonstrates time-dependent immune cell infiltration in human ex-vivo burn-injured skin. Sci Rep 2021;11:21249. [Crossref] [PubMed]
- Koguchi-Yoshioka H, Watanabe R, Matsumura Y, et al. Serum lactate dehydrogenase level as a possible predictor of treatment preference in psoriasis. J Dermatol Sci 2021;103:109-15. [Crossref] [PubMed]
- Hisamoto T, Suga H, Yoshizaki-Ogawa A, et al. Increased Serum Levels of Tumor Necrosis Factor-like Ligand 1A in Atopic Dermatitis. Int J Mol Sci 2023;24:1813. [Crossref] [PubMed]
- Yu L, Li L. Potential biomarkers of atopic dermatitis. Front Med (Lausanne) 2022;9:1028694. [Crossref] [PubMed]
- Morishima Y, Kawashima H, Takekuma K, et al. Changes in serum lactate dehydrogenase activity in children with atopic dermatitis. Pediatr Int 2010;52:171-4. [Crossref] [PubMed]
- Kato A, Kamata M, Ito M, et al. Higher baseline serum lactate dehydrogenase level is associated with poor effectiveness of dupilumab in the long term in patients with atopic dermatitis. J Dermatol 2020;47:1013-9. [Crossref] [PubMed]
- Tas F, Oguz H, Argon A, et al. The value of serum levels of IL-6, TNF-alpha, and erythropoietin in metastatic malignant melanoma: serum IL-6 level is a valuable prognostic factor at least as serum LDH in advanced melanoma. Med Oncol 2005;22:241-6. [Crossref] [PubMed]
- Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol 2010;163:238-56. [Crossref] [PubMed]
- Scarisbrick JJ. Staging and management of cutaneous T-cell lymphoma. Clin Exp Dermatol 2006;31:181-6. [Crossref] [PubMed]
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472-92.
- Livesey A, Garty F, Shipman AR, et al. Lactate dehydrogenase in dermatology practice. Clin Exp Dermatol 2020;45:539-43. [Crossref] [PubMed]
- Jiang Y, Li X, Zhou W, et al. Clinical significance of serum ferritin in patients with systemic sclerosis. J Clin Lab Anal 2022;36:e24597. [Crossref] [PubMed]
- Erlij D, Cuellar MC, Badilla N, et al. Lymphadenopathies in patients with rheumatic diseases. Review of 19 cases. Rev Med Chil 2020;148:320-6. [Crossref] [PubMed]
- Yamada H, Kaneko Y, Tamai H, et al. Biomarkers for disease flare in patients with adult-onset Still's disease undergoing treatment with tocilizumab. Rheumatology (Oxford) 2020;59:440-2. [Crossref] [PubMed]
- Pejovic M, Stankovic A, Mitrovic DR. Lactate dehydrogenase activity and its isoenzymes in serum and synovial fluid of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 1992;19:529-33. [PubMed]
- Wang W, Gong F, Zhu W, et al. Macrophage activation syndrome in Kawasaki disease: more common than we thought? Semin Arthritis Rheum 2015;44:405-10. [Crossref] [PubMed]
- Chen Z, Lin L, Yang W, et al. Clinical characteristics and prognostic risk factors of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV). Int Immunopharmacol 2020;87:106819. [Crossref] [PubMed]
- Matsushita S, Sada K, Manabe A, et al. Elevated White Blood Cell Count and Lactate Dehydrogenase Levels Are Important Markers for Diagnosing Relapse of Adult-onset Still's Disease under Tocilizumab Use. Intern Med 2022;61:3743-7. [Crossref] [PubMed]
- Ayano M, Arinobu Y, Tsukamoto H, et al. Shoulder ultrasound and serum lactate dehydrogenase predict inadequate response to glucocorticoid treatment in patients with polymyalgia rheumatica. Rheumatol Int 2020;40:1101-9. [Crossref] [PubMed]
- Miyamoto T, Oguma Y, Sato Y, et al. Elevated Creatine Kinase and Lactic Acid Dehydrogenase and Decreased Osteocalcin and Uncarboxylated Osteocalcin are Associated with Bone Stress Injuries in Young Female Athletes. Sci Rep 2018;8:18019. [Crossref] [PubMed]
- Demirel E, Şahin A. Predictive Value of Blood Parameters and Comorbidities on Three-Month Mortality in Elderly Patients With Hip Fracture. Cureus 2021;13:e18634. [Crossref] [PubMed]
- Wukich DK, Callaghan JJ, Graeber GM, et al. Operative treatment of acute hip fractures: its effect on serum creatine kinase, lactate dehydrogenase and their isoenzymes. J Trauma 1989;29:375-9. [Crossref] [PubMed]
- Whyte MP, Kempa LG, McAlister WH, et al. Elevated serum lactate dehydrogenase isoenzymes and aspartate transaminase distinguish Albers-Schönberg disease (Chloride Channel 7 Deficiency Osteopetrosis) among the sclerosing bone disorders. J Bone Miner Res 2010;25:2515-26. [Crossref] [PubMed]
- Chu H, Huang C, Dong J, et al. Lactate Dehydrogenase Predicts Early Hematoma Expansion and Poor Outcomes in Intracerebral Hemorrhage Patients. Transl Stroke Res 2019;10:620-9. [Crossref] [PubMed]
- Parakh N, Gupta HL, Jain A. Evaluation of enzymes in serum and cerebrospinal fluid in cases of stroke. Neurol India 2002;50:518-9. [PubMed]
- Jin XX, Fang MD, Hu LL, et al. Elevated lactate dehydrogenase predicts poor prognosis of acute ischemic stroke. PLoS One 2022;17:e0275651. [Crossref] [PubMed]
- Yilmaz M, Tekten BO. Serum prolactin level and lactate dehydrogenase activity in patients with epileptic and nonepileptic seizures: A cross-sectional study. Medicine (Baltimore) 2021;100:e27329. [Crossref] [PubMed]
- Nand N, Sharma M, Saini DS. Evaluation of lactic dehydrogenase in cases of meningitis. Indian J Med Sci 1993;47:96-100. [PubMed]
- Zhang L, Liu K, Su Q, et al. Clinical features of the first critical case of acute encephalitis caused by the avian influenza A (H5N6) virus. Emerg Microbes Infect 2022;11:2437-46. [Crossref] [PubMed]
- Gao B, Liu FL, Zhao B. Association of degree and type of edema in posterior reversible encephalopathy syndrome with serum lactate dehydrogenase level: initial experience. Eur J Radiol 2012;81:2844-7. [Crossref] [PubMed]
- Vargas M, Servillo G, Striano P. Serum lactate dehydrogenase as early marker of posterior reversible encephalopathy syndrome: keep your eyes open. Anaesth Intensive Care 2012;40:570-1. [PubMed]
- Rabow L, Tibbling G. Serum enzyme activity of hydroxybutyric dehydrogenase (HBD) and heat inactivated lactate dehydrogenase (LD) after operations on intracranial aneurysms. Acta Neurochir (Wien) 1975;32:199-207. [Crossref] [PubMed]
- Rabow L, Tibbling G. Serum activities of hydroxybutyrate dehydrogenase (HBD) and the isoenzymes of lactate dehydrogenase (LD) as an index of traumatic brain injury. Acta Neurochir (Wien) 1977;37:245-61. [Crossref] [PubMed]
- Bodor GS. Biochemical Markers of Myocardial Damage. EJIFCC 2016;27:95-111. [PubMed]
- Panteghini M. IFCC Committee on Standardization of Markers of Cardiac Damage: premises and project presentation. International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med 1998;36:887-93. [Crossref] [PubMed]
- Caldara G. Lactic acid dehydrogenase in the diagnosis of rheumatic carditis. Arch Sci Med (Torino) 1969;126:659-62. [PubMed]
- Akhtar MS, Hassan Q, Afzal O, et al. Comparative Efficacy of Levosimendan, Ramipril, and Sacubitril/ Valsartan in Isoproterenol-induced Experimental Heart Failure: A Hemodynamic and Molecular Approach. Curr Mol Pharmacol 2023;16:629-39. [Crossref] [PubMed]
- Fang XX, Shen LL, Yao WB, et al. Correlation between lactate dehydrogenase and QTc interval prolongation in maintenance hemodialysis patients: a cross-sectional study. Ann Palliat Med 2022;11:3444-54. [Crossref] [PubMed]
- Li M, Tang M, Zhao C, et al. Prognostic Potential of Pulmonary Hypertension in Patients with Hematologic Malignancy. Adv Ther 2023;40:4792-804. [Crossref] [PubMed]
- Hu HJ, Zhang C, Tang ZH, et al. Regulating the Warburg effect on metabolic stress and myocardial fibrosis remodeling and atrial intracardiac waveform activity induced by atrial fibrillation. Biochem Biophys Res Commun 2019;516:653-60. [Crossref] [PubMed]
- Binder J, Palmrich P, Kalafat E, et al. Longitudinal assessment of angiogenic markers in prediction of adverse outcome in women with confirmed pre-eclampsia. Ultrasound Obstet Gynecol 2023;62:843-51. [Crossref] [PubMed]
- Širáková A, Toušek P, Bednář F, et al. Intravascular haemolysis after transcatheter aortic valve implantation with self-expandable prosthesis: incidence, severity, and impact on long-term mortality. Eur Heart J Suppl 2020;22:F44-50. [Crossref] [PubMed]
- Wang X, Zhang J, Qian J, et al. The Regulatory Mechanism and Effect of Receptor-Interacting Protein Kinase 3 on Phenylephrine-Induced Cardiomyocyte Hypertrophy. J Cardiovasc Pharmacol 2022;80:236-50. [Crossref] [PubMed]
- Casós K, Ferrer-Curriu G, Soler-Ferrer P, et al. Response of the human myocardium to ischemic injury and preconditioning: The role of cardiac and comorbid conditions, medical treatment, and basal redox status. PLoS One 2017;12:e0174588. [Crossref] [PubMed]
- Wachsmuth ED. Localization of lactate dehydrogenase isozymes in human muscle tissues by the mixed aggregation immunocytochemical technique. Histochemistry 1979;60:249-54. [Crossref] [PubMed]
- Suh DS, Song YJ, Roh HJ, et al. Preoperative Blood Inflammatory Markers for the Differentiation of Uterine Leiomyosarcoma from Leiomyoma. Cancer Manag Res 2021;13:5001-11. [Crossref] [PubMed]
- Glorie N, Baert T. Circulating Protein Biomarkers to Differentiate Uterine Sarcomas from Leiomyomas. Anticancer Res 2019;39:3981-9. [Crossref] [PubMed]
- Köhler G, Vollmer M, Nath N, et al. Benign uterine mass-discrimination from leiomyosarcoma by a preoperative risk score: a multicenter cohort study. Arch Gynecol Obstet 2019;300:1719-27. [Crossref] [PubMed]
- Sakamoto K, Ohata M, Hashimoto K, et al. Two cases of alcoholics associated with rhabdomyolysis and acute renal failure. Nihon Arukoru Yakubutsu Igakkai Zasshi 2002;37:505-12. [PubMed]
- Gofshteyn JS, Mansfield L, Spitznagle J, et al. Association of Juvenile Dermatomyositis Disease Activity With the Expansion of Blood Memory B and T Cell Subsets Lacking Follicular Markers. Arthritis Rheumatol 2023;75:1246-61. [Crossref] [PubMed]
- Sadek AA, Mahmoud SM, El-Aal MA, et al. Evaluation of cardiac functions in children with Duchenne Muscular Dystrophy: A prospective case-control study. Electron Physician 2017;9:5732-9. [Crossref] [PubMed]
- Mieszkowski J, Stankiewicz BE, Kochanowicz A, et al. Remote Ischemic Preconditioning Reduces Marathon-Induced Oxidative Stress and Decreases Liver and Heart Injury Markers in the Serum. Front Physiol 2021;12:731889. [Crossref] [PubMed]
- Biguetti CC, Junior JFS, Fiedler MW, et al. The toxic effects of chloroquine and hydroxychloroquine on skeletal muscle: a systematic review and meta-analysis. Sci Rep 2021;11:6589. [Crossref] [PubMed]
- Hassouneh R, Shen S, Lee O, et al. Severe Vitamin B12 Deficiency Mimicking Microangiopathic Hemolytic Anemia. J Hematol 2021;10:202-5. [Crossref] [PubMed]
- Jaswal TS, Mehta HC, Gupta V, et al. Serum lactate dehydrogenase in diagnosis of megaloblastic anaemia. Indian J Pathol Microbiol 2000;43:325-9. [PubMed]
- Najim OA, Hassan MK. Lactate dehydrogenase and severity of pain in children with sickle cell disease. Acta Haematol 2011;126:157-62. [Crossref] [PubMed]
- Stankovic Stojanovic K, Lionnet F. Lactate dehydrogenase in sickle cell disease. Clin Chim Acta 2016;458:99-102. [Crossref] [PubMed]
- Ikeda S, Sugihara T, Kihara T, et al. Chronic Active Epstein-Barr Virus Infection Indistinguishable from Autoimmune Hepatitis: A Case Report. Yonago Acta Med 2022;65:160-5. [Crossref] [PubMed]
- Kaya A, Kuku İ, Erkurt MA, et al. The effect of thrombocytapheresis on hemogram and biochemistry parameters in patients with essential thrombocytemia. Transfus Clin Biol 2023;30:421-5. [Crossref] [PubMed]
- Miyashita N, Ohashi K, Fujita M, et al. Prognostic factors in patients in the terminal phase of haematological malignancies who are receiving home medical care. Br J Haematol 2023;201:290-301. [Crossref] [PubMed]
- Lee AJ, Kim SG, Nam JY, et al. Clinical features and outcomes of JAK2 V617F-positive polycythemia vera and essential thrombocythemia according to the JAK2 V617F allele burden. Blood Res 2021;56:259-65. [Crossref] [PubMed]
- el Barzouhi A, van Buren M, van Nieuwkoop C. Renal and Splenic Infarction in a Patient with Familial Hypercholesterolemia and Previous Cerebral Infarction. Am J Case Rep 2018;19:1463-6. [Crossref] [PubMed]
- Fishman J, Kuranz S, Yeh MM, et al. Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network. Hematol Rep 2023;15:266-82. [Crossref] [PubMed]
- Tsuchiya J. Some problems in the laboratory findings in multiple myeloma. Nihon Ketsueki Gakkai Zasshi 1989;52:1273-8. [PubMed]
- Chen W, Zhang Q, Li M, et al. Clinical diagnostic value of serum soluble IL-2 receptor for stage I sarcoidosis in benign isolated mediastinal and hilar lymphadenopathy. Clin Chim Acta 2023;545:117372. [Crossref] [PubMed]
- Ye Y, Chen M, Chen X, et al. Clinical Significance and Prognostic Value of Lactate Dehydrogenase Expression in Cervical Cancer. Genet Test Mol Biomarkers 2022;26:107-17. [Crossref] [PubMed]
- Li J, Wu MF, Lu HW, et al. Pretreatment serum lactate dehydrogenase is an independent prognostic factor for patients receiving neoadjuvant chemotherapy for locally advanced cervical cancer. Cancer Med 2016;5:1863-72. [Crossref] [PubMed]
- Birbas E, Kanavos T, Gkrozou F, et al. Ovarian Masses in Children and Adolescents: A Review of the Literature with Emphasis on the Diagnostic Approach. Children (Basel) 2023;10:1114. [Crossref] [PubMed]
- Karim SMH, Fattah CN. The correlation between women's various gynaecological diseases to ovarian cancer in Sulaimaniyah City, Iraq. J Int Med Res 2023;51:3000605231158949. [Crossref] [PubMed]
- Shinmura H, Yoneyama K, Harigane E, et al. Use of tumor markers to distinguish endometriosis-related ovarian neoplasms from ovarian endometrioma. Int J Gynecol Cancer 2020;30:831-6. [Crossref] [PubMed]
- Khan TT, Mirza AB, Zahid R, et al. Antibody-mediated rejection: importance of lactate dehydrogenase and neutrophilia in early diagnosis. Saudi J Kidney Dis Transpl 2011;22:525-30. [PubMed]
- Sakhinia F, Ollerenshaw R, Wallace D. Mechanical stress-related haemolysis in a paediatric haemodialysis patient. BMJ Case Rep 2022;15:e245248. [Crossref] [PubMed]
- Huang Z, Hu Y, Chen B, et al. Clinical significance of intrarenal vascular lesions in non-hypertensive patients with IgA nephropathy. J Nephrol 2023;36:429-40. [Crossref] [PubMed]
- Alkhatib MH, Kant S, Menez S, et al. Thrombotic microangiopathy versus class IV lupus nephritis in systemic lupus erythematosus. J Nephrol 2021;34:1907-13. [Crossref] [PubMed]
- Lee DY, Kim JY, Ahn E, et al. Associations between local acidosis induced by renal LDHA and renal fibrosis and mitochondrial abnormalities in patients with diabetic kidney disease. Transl Res 2022;249:88-109. [Crossref] [PubMed]
- Korzets Z, Plotkin E, Bernheim J, et al. The clinical spectrum of acute renal infarction. Isr Med Assoc J 2002;4:781-4. [PubMed]
- Kiker JD, Woodside JR, Reed WP, et al. Urinary lactic dehydrogenase and serum C-reactive protein as means of localizing the site of urinary tract infection in patients with ileal conduits. J Urol 1982;128:749-51. [Crossref] [PubMed]
- Yang F, Li D, Di Y, et al. Pretreatment Serum Cystatin C Levels Predict Renal Function, but Not Tumor Characteristics, in Patients with Prostate Neoplasia. Biomed Res Int 2017;2017:7450459. [Crossref] [PubMed]
- Neĭmark AI, Fidirkin AV, Zviagintsev EN, et al. The diagnostic significance of enzymuria in assessing kidney function in patients with urolithiasis. Urol Nefrol (Mosk) 1997;5-7. [PubMed]
- Wei W, Cai Z, Chen L, et al. Short-term prognostic models for severe acute kidney injury patients receiving prolonged intermittent renal replacement therapy based on machine learning. BMC Med Inform Decis Mak 2023;23:133. [Crossref] [PubMed]
- Chen J, Wu F, Cao Y, et al. The novel role of LDHA/LDHB in the prognostic value and tumor-immune infiltration in clear cell renal cell carcinoma. PeerJ 2023;11:e15749. [Crossref] [PubMed]
- Liu TY, Lee WJ, Tsai CM, et al. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Neonatol 2018;59:501-6. [Crossref] [PubMed]
- Ding CY, Peng L, Lin YX, et al. Elevated Lactate Dehydrogenase Level Predicts Postoperative Pneumonia in Patients with Aneurysmal Subarachnoid Hemorrhage. World Neurosurg 2019;129:e821-30. [Crossref] [PubMed]
- Kavvasoglu B, Kavvasoglu GH. Predictive value of clinical and laboratory parameters in differentiating hypertensive pulmonary edema and acute pulmonary embolism. Eur Rev Med Pharmacol Sci 2023;27:7255-63. [PubMed]
- He Z, Bi W, Lang Z, et al. Comparative study on electrocardiograms and serological examinations of acute pulmonary embolism and acute non-ST elevation myocardial infarction. Ann Noninvasive Electrocardiol 2022;27:e12920. [Crossref] [PubMed]
- Pan R, Ren Y, Li Q, et al. Neutrophil-lymphocyte ratios in blood to distinguish children with asthma exacerbation from healthy subjects. Int J Immunopathol Pharmacol 2023;37:3946320221149849. [Crossref] [PubMed]
- Huang L, Lu Z, Zhou X, et al. U-Shaped Relationship Between Serum Lactate Dehydrogenase with All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis 2023;18:305-16. [Crossref] [PubMed]
- Okamoto S, Tsuboi H, Noma H, et al. Predictive Factors for Pneumomediastinum During Management of Connective Tissue Disease-related Interstitial Lung Disease: A Retrospective Study. Intern Med 2021;60:2887-97. [Crossref] [PubMed]
- Frenkel A, Shiloh A, Azulay B, et al. The role of lactate dehydrogenase in hospitalized patients, comparing those with pulmonary versus non-pulmonary infections: A nationwide study. PLoS One 2023;18:e0283380. [Crossref] [PubMed]
- Komolafe O, Pereira SP, Davidson BR, et al. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database Syst Rev 2017;4:CD012645. [Crossref] [PubMed]
- Sachs SM, Morton JH, Schwartz SI. Acute mesenteric ischemia. Surgery 1982;92:646-53. [PubMed]
- Ceylan C, Baran NT, Kocaaslan H, et al. A new model for prediction of bowel gangrene in sigmoid volvulus. Ulus Travma Acil Cerrahi Derg 2023;29:471-6. [Crossref] [PubMed]
- Price JR, Hagrass H, Filip AB, et al. LDH and the MELD-LDH in Severe Acute Liver Injury and Acute Liver Failure: Preliminary Confirmation of a Novel Prognostic Score for Risk Stratification. J Appl Lab Med 2023;8:504-13. [Crossref] [PubMed]
- Ocak T, Gülten M. Retrospective Investigation of Factors Affecting Mortality in Spontaneous Bacterial Peritonitis. Euroasian J Hepatogastroenterol 2023;13:5-9. [Crossref] [PubMed]
- Teniola D, Ayoola EA, Arigbabu AO. Lactic dehydrogenase levels in patients with duodenal ulcer, gastric ulcer, gastric polys and gastric carcinoma. Scand J Gastroenterol Suppl 1986;124:169-78. [Crossref] [PubMed]
- McGrowder DA, Fraser YP, Gordon L, et al. Serum creatine kinase and lactate dehydrogenase activities in patients with thyroid disorders. Niger J Clin Pract 2011;14:454-9. [Crossref] [PubMed]
- Roti E, Bandini P, Robuschi G, et al. Serum concentrations of myoglobin, creatine kinase, lactate dehydrogenase and cardiac isoenzymes in euthyroid, hypothyroid and hyperthyroid subjects. Ric Clin Lab 1980;10:609-17. [Crossref] [PubMed]
- Zhong XH, Howard BD. Phosphotyrosine-containing lactate dehydrogenase is restricted to the nuclei of PC12 pheochromocytoma cells. Mol Cell Biol 1990;10:770-6. [PubMed]
- Hsieh YS, Yeh MC, Lin YY, et al. Is the level of serum lactate dehydrogenase a potential biomarker for glucose monitoring with type 2 diabetes mellitus? Front Endocrinol (Lausanne) 2022;13:1099805. [Crossref] [PubMed]
- Sato T, Hiramatsu R, Iwaoka T, et al. Changes of platelets, serum lactic dehydrogenase, gamma-glutamyltranspeptidase, choline esterase and creatine phosphokinase levels in patients with Cushing's syndrome. Tohoku J Exp Med 1984;142:195-200. [Crossref] [PubMed]
- Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark 2017;19:353-63. [Crossref] [PubMed]
- Bien E, Rapala M, Krawczyk M, et al. The serum levels of soluble interleukin-2 receptor alpha and lactate dehydrogenase but not of B2-microglobulin correlate with selected clinico-pathological prognostic factors and response to therapy in childhood soft tissue sarcomas. J Cancer Res Clin Oncol 2010;136:293-305. Erratum in: J Cancer Res Clin Oncol 2010;136:307. [Crossref] [PubMed]
- Boran N, Kayikçioğlu F, Yalvaç S, et al. Significance of serum and peritoneal fluid lactate dehydrogenase levels in ovarian cancer. Gynecol Obstet Invest 2000;49:272-4. [Crossref] [PubMed]
- Fitzgerald RT, Wright SM, Samant RS, et al. Elevation of serum lactate dehydrogenase at posterior reversible encephalopathy syndrome onset in chemotherapy-treated cancer patients. J Clin Neurosci 2014;21:1575-8. [Crossref] [PubMed]
- Shi M, Lin J, Wei W, et al. Machine learning-based in-hospital mortality prediction of HIV/AIDS patients with Talaromyces marneffei infection in Guangxi, China. PLoS Negl Trop Dis 2022;16:e0010388. [Crossref] [PubMed]
- Nakakubo S, Unoki Y, Kitajima K, et al. Serum Lactate Dehydrogenase Level One Week after Admission Is the Strongest Predictor of Prognosis of COVID-19: A Large Observational Study Using the COVID-19 Registry Japan. Viruses 2023;15:671. [Crossref] [PubMed]
- Plagemann PG, Moennig V. Lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus: a new group of positive-strand RNA viruses. Adv Virus Res 1992;41:99-192. [Crossref] [PubMed]
- Nair RK, Rao KA, Mukherjee D, et al. Acute kidney injury due to acute cortical necrosis following vivax malaria. Saudi J Kidney Dis Transpl 2019;30:960-3. [Crossref] [PubMed]
- Dodo M, Kitamura H, Shima H, et al. Lactate dehydrogenase C is required for the protein expression of a sperm-specific isoform of lactate dehydrogenase A. J Biochem 2019;165:323-34. [Crossref] [PubMed]
- OMIM. Accessed 20.09.2023. Available online: https://www.omim.org/search?index=entry&start=1&limit=10&sort=score+desc%2C+prefix_sort+desc&search=%22lactate+dehydrogenase%22
- Anai T, Urata K, Tanaka Y, et al. Pregnancy complicated with lactate dehydrogenase M-subunit deficiency: the first case report. J Obstet Gynaecol Res 2002;28:108-11. [Crossref] [PubMed]
- He S, Bremme K, Kallner A, et al. Increased concentrations of lactate dehydrogenase in pregnancy with preeclampsia: a predictor for the birth of small-for-gestational-age infants. Gynecol Obstet Invest 1995;39:234-8. [Crossref] [PubMed]
- Bera S, Gupta S, Saha S, et al. Study of liver enzymes especially lactate dehydrogenase to predict foetal outcome in pregnancy induced hypertension. Sch J App Med Sci 2014;2:1569-72.
- Zhang X, Chen Y, Salerno S, et al. Prediction of intrahepatic cholestasis of pregnancy in the first 20 weeks of pregnancy. J Matern Fetal Neonatal Med 2022;35:6329-35. [Crossref] [PubMed]
- van der Sluijs Veer G. Reference values for lactate dehydrogenase in the serum during childhood and puberty (author's transl). J Clin Chem Clin Biochem 1980;18:305-6. [PubMed]
- Colantonio DA, Kyriakopoulou L, Chan MK, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012;58:854-68. [Crossref] [PubMed]
Cite this article as: Shipman AR, Bahrani S, Shipman KE. Investigative algorithms for disorders affecting plasma lactate dehydrogenase: a narrative review. J Lab Precis Med 2024;9:15.


