
Circulating calprotectin in rheumatoid arthritis: unravelling the impact of (pre-)analytical confounders on meta-analysis results
Highlight box
Key findings
• Inter-study heterogeneity on the clinical evidence of circulating calprotectin (cCLP) in rheumatoid arthritis (RA) is, besides analytical issues, mainly caused by pre-analytical confounders.
What is known and what is new?
• cCLP is a promising diagnostic and prognostic biomarker in (pre-)RA.
• Pre-analytically, cCLP levels depend on the sample matrix, time to centrifugation and conservation temperature.
• Only a limited number of studies on cCLP in RA encounter pre-analytic variables in their study protocols.
What is the implication, and what should change now?
• Adherence to pre-analytical recommendations is primordial in studies on cCLP in RA and significantly reduces inter-study heterogeneity.
Introduction
Background
Rheumatoid arthritis (RA) is a chronic and systemic autoimmune disease more commonly affecting women at an incidence of 1 in 150 (1,2). RA is mainly characterized by synovitis, which can potentially result in irreversible dysfunction and deformation of the affected joints, reduction of quality of life and increased mortality (3). Thus, proper diagnosis and treatment with disease modifying anti-rheumatic drugs (DMARD) are essential to prevent or slow down disease progression (4,5). However, identifying a patient with RA among patients with symptoms of arthritis remains challenging (6).
Therefore, clinicians rely on serological biomarkers to assist in RA diagnosis. Serum elevations of antibodies to citrullinated protein antigens (ACPA) and rheumatoid factor (RF) are hallmarks in RA. As ACPA and/or RF may proceed clinical RA diagnosis up to 10 years (7), additional biomarkers are warranted to improve the prediction of transition from early RA to clinically overt disease (8). In addition to ACPA and RF, acute-phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are included as serological markers in the current American College of Rheumatology (ACR)/the European Alliance of Associations for Rheumatology (EULAR) RA classification criteria (9).
Rationale and knowledge gap
More recently, circulating calprotectin (cCLP) has been proposed as an alternative diagnostic and prognostic biomarker in neutrophil-related inflammation (10). Calprotectin (CLP) is a heterodimer of two proteins (S100A8 and S100A9) produced by monocytes and neutrophils in circulation and tissue in response to inflammation (11,12). Since CLP is released locally, cCLP systemically mirrors local disease activity in neutrophil-related inflammatory diseases (10,13). In RA patients, increased CLP concentrations are found in synovial fluid (14), in parallel with cCLP blood levels (15), and correlate with disease activity (16-18) and with ultrasound synovitis scores (19-21). In addition, cCLP levels positively correlate with RF and ACPA titers (19,22,23) and are independently associated with radiographic progression and future erosive damage in RA (20,21).
Despite being a promising biomarker, recent meta-analyses investigating the clinical evidence of cCLP in RA patients, describe an important inter-study heterogeneity (16-18), hampering adequate inter-study comparison. Study heterogeneity can be multifactorial. Analytically, cCLP reference values depend on (I) the sample type used for blood collection, i.e., significantly higher cCLP levels are observed in serum than in plasma (24,25), and (II) the (commercial) immunoassay used for cCLP measurement (24,25). Pre-analytically, cCLP levels significantly rise in vitro due to delayed sample centrifugation and, depending on the sample type, increase (serum, citrate, or lithium heparin plasma) or decrease [ethylenediaminetetraacetic acid (EDTA) plasma] due to prolonged storage at 30 ℃ or room temperature. Therefore, strict pre-analytical recommendations have been suggested for cCLP testing, with pre-centrifugation time being more stringent for serum (<2 hours) than for plasma (<6 hours) (24,26).
Objective
In the current study we aimed to replicate recent meta-analyses and investigate the impact of pre-analytical and analytical confounders on the inter-study heterogeneity of available publications from January 2000–February 2023. We present this article in accordance with the PRISMA reporting checklist (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-79/rc) (27).
Methods
Search strategy
A systematic literature search was performed in the electronic databases Medline, Embase and Cochrane (March 23rd, 2023) for studies that examine CLP levels in RA patients and healthy controls (HCs). The search terms used were: “Leukocyte L1 antigen complex”, “rheumatoid arthritis”, “rheumatic disease”, “circulating calprotectin”, “blood calprotectin”, “calgranulin A/B”, “MRP8/14”, “S100A8/A9”. A time filter was applied, including articles published from the year 2000 onwards.
Inclusion/exclusion criteria
After removing duplicates from the different databases, article selection was done in two steps. First, titles and abstracts were screened by a single reviewer.
Inclusion criteria were: (I) articles written in English, with full text available for analysis and a study population consisting of RA patients at time-point of diagnosis, included according to ACR and/or EULAR criteria (9,27) and a HC cohort; (II) articles clearly specifying sample type (serum/plasma) and CLP measurement method, providing quantitative CLP data [mean ± standard deviation (SD), or median and interquartile range (IQR)]. Exclusion criteria were (I) abstracts, letters, case reports, reviews, articles investigating rheumatic diseases other than RA or (II) investigating CLP in synovial fluid only and articles with overlapping/insufficient data.
In a second step, full-text articles were further assessed for eligibility by two independent reviewers using the National Institute of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies to reduce the risk of bias. Discrepancies were resolved by consensus.
Data extraction
The following data was extracted from the included studies: first author, year of publication, study design, number of RA patients and disease status, number and type (i.e., healthy, rheumatologic disease, etc.) of control patients, sample type, CLP measurement method, mean and standard deviation of CLP baseline values, pre-centrifugation time and storage temperature of CLP samples. When data were presented as median and IQR, the mean and SD were calculated using previously described formulas (28). For studies evaluating multiple cCLP assays on the same sample cohorts, the specific HC and RA results related to every assay were considered as a unique data set, resulting in an assay-specific standardized mean difference (SMD) value.
Statistical analysis
To examine potential sources of heterogeneity in the meta-analysis, subgroup analysis was performed using pre-analytical and analytical confounders as variables. To compare cCLP values between these groups in terms of standardized scores, data were presented as SMD with 95% confidence interval (CI). The magnitude of the SMD was interpreted as follows: 0.2–0.49 small effect, 0.5–0.79 medium effect, ≥0.8 large effect. Heterogeneity among studies was assessed using the inconsistency index (I2). A value >50% indicated significant heterogeneity. The pooled effect was estimated by using both the fixed and random effects models. When heterogeneity is high, the fixed effects model, which assumes that all studies come from a common population and that the effect size is not significantly different among the different trials, might be invalid. Under these circumstances the random effects model, which incorporates both the random variation within and variation between the different studies, may be more appropriate. For each meta-analysis, heterogeneity was assessed using the Cochran’s Q and the I2 statistic. A P value <0.05 was considered statistically significant. All data analyses were performed in MEDCALC® Statistical Software version 22.021 (MedCalc Software Ltd., Ostend, Belgium), including the Egger’s test and Begg’s rank test to evaluate the publication bias.
Results
Studies included in the meta-analysis
A flow diagram of the article selection is shown in Figure 1. Our search resulted in a total of 646 titles from the three databases. Articles including incomplete or duplicate data, non-diagnostic RA patients or non-HC cohorts were excluded, together with meta-analysis, systematic reviews and letters to the editor. After removal of duplicates and screening of title and abstract, 44 titles were retrieved for full-text screening. Eventually, 35 articles were eligible for inclusion in our study. Only studies comparing HC and RA patients were selected and resulted in 18 titles included in quantitative synthesis for meta-analysis. A total of 21 data sets were included as one title compared four different assays using the same HC and RA patient group. Of the 18 included titles, 11 had a cross-sectional design, 6 had a longitudinal design and 1 study had both (Appendix 1). Appendix 1 provides an overview of the studies included in the meta-analysis, including the assays used, the consideration of any pre-analytical confounder and the number of samples included in the study cohorts.
Meta-analysis of cCLP levels in RA versus HCs
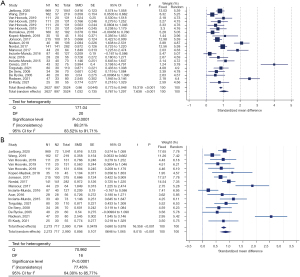
In total, cCLP was measured in 2,627 RA and 897 HC. CLP levels in RA patients were significantly higher compared to HC (SMD =1.032, 95% CI: 0.767–1.297, P<0.001) (Figure 2A). Significant (P<0.0001) heterogeneity was observed (Cochran’s Q =171.04; I2 statistic =88.31%; 95% CI: 83.52–91.71%) between all studies. CLP analyses were performed using 14 different cCLP assays, comprising both in-house and commercial assays. Outliers were observed for studies using the Bühlmann assay. After exclusion of the four studies based on the Bühlmann assay, significant heterogeneity on the meta-analysis results remained (Cochran’s Q =70.992; I2 statistic =77.46%; 95% CI: 64.30–85.77) (Figure 2B).
Impact of pre-analytical confounders on meta-analysis results
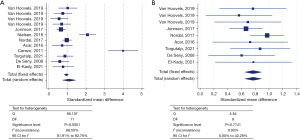
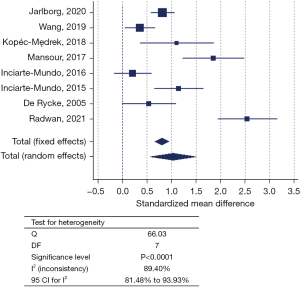
In 12 out of 21 included datasets, adherence to pre-analytical conditions (i.e., time to centrifugation and/or sample storage temperature) was specified (Table S1). Conservation temperature of the samples ranged from −20 ℃ to −140 ℃, with −80 ℃ being the most frequent (n=7/12) and pre-centrifugation time intervals ranged from ‘immediately’ to ≤2 hours (Table S1). Subgroup analysis of these 12 datasets still showed significant heterogeneity (Cochran’s Q =96.107; I2 statistic =88.55%; 95% CI: 81.91–92.76%) (Figure 3A), mainly caused by the datasets using the Bühlmann assay (Table S1). Only after exclusion of the latter datasets, the datasets of studies adhering to pre-analytical recommendations (n=9) showed absence of heterogeneity (Q =4.84; I2 statistic =0.00; 95% CI: 0.00–42.28) (Figure 3B). Nevertheless, still eight different cCLP assays were encountered, together with a total sample cohort of 856 RA patients and 425 HC. Regardless of the sample type, higher cCLP concentrations were obtained for the RA versus HC in both subgroups. In the meta-analysis comprising the datasets of the studies non-compliant to pre-analytical recommendations (n=8), significant inter-study heterogeneity persisted (Q =66.03; I2 statistic =89.40%; 95% CI: 81.48–93.93%) (Figure 4). In 9 out of 21 included studies, time to centrifugation was not specified, neither storage temperature in 6 out of these 9 studies. The total SMD for samples with adherence to centrifugation time (n=12) is 1.094 and 0.954 for samples without any centrifugation time mentioned. When excluding results obtained with Bühlmann assay, the values for SMD declined to 0.769 and 1.035, respectively. For samples for which storage temperature was mentioned (n=15), the total SMD was 0.846 compared to a total SMD of 0.892 for samples without any storage temperature information.
Publication bias
For every meta-analysis performed, publication bias was evaluated using the Egger’s test and Begg’s rank test (MedCalc Software version 22.021). Both tests did not demonstrate significant publication bias for any of the meta-analyses performed (Appendix 1).
Discussion
In the current ACR/EULAR RA classification criteria both the traditional RA antibodies, RF and ACPA, as well as the acute-phase reactants ESR and CRP are included as serological biomarkers (9). Already in 2015, Hurnakova et al. described cCLP as a more sensitive biomarker of inflammatory activity in RA than ESR and CRP (29,30). In the recent years, the insights on the diagnostic and prognostic value of cCLP in RA only increased (16-23). At presentation, CRP and ESR can be normal in 35–40% of RA patients, whilst cCLP has shown to be already elevated in early stages of the disease (13,18). In RA patients with moderate to high disease activity and normal CRP levels, cCLP more accurately reflected inflammation (20,29,30). Furthermore, as CRP production in the liver is stimulated by interleukin 6 (IL-6), cCLP proved to be a more valuable inflammatory biomarker than CRP in RA patients treated with IL-6 blocking agents (31). Finally, suiting this era of biological treatments, wherein the clinician’s goal is to shift from RA diagnosis to RA prevention (8), Bettner et al. recently showed that adding elevated cCLP levels to RF and ACPA positivity resulted in a high predictive value (53%) for the development of RA within 3 years or less (32).
Despite being a promising biomarker, recent meta-analyses on the diagnostic potential of cCLP levels in RA showed substantial inter-study variability (16-18). Although cCLP is relatively stable and easily measurable in blood (13), it occurs in different conformational complexes, e.g., S100A8 and S100A9 monomers and, in the presence of calcium ions, heterodimers and heterotetramers (11,33,34). The presence of the different conformational structures impacts cCLP diagnostics.
Firstly, a sample matrix effect has been described revealing higher cCLP levels in serum than in plasma samples (24-26,35-37). This matrix effect is mainly due to the in vitro release of CLP by neutrophils after sampling and during coagulation in serum tubes. Therefore, sample matrix dependent reference values should be used to correctly interpret cCLP levels (36).
Secondly, not only the relative ratio of the different cCLP structures, but also their stability has shown to be dependent on, besides the sample matrix, also the storage conditions. A lag time of 2 hours versus 30 minutes between sample collection and centrifugation did not significantly affect cCLP levels, in contrast to a longer time to centrifugation (e.g., 6 hours or more) (24,25,36). cCLP levels revealed to decrease in EDTA plasma, in contrast to an increase in the other sample types after prolonged storage at 30 ℃ or room temperature (24,26). This is presumably caused by the higher proteolytic vulnerability of cCLP monomers (34), which are expected to be the most predominant conformational structure in EDTA plasma. To minimize the in vitro effects on cCLP levels, pre-analytical recommendations have been suggested (24,26,36), being stricter for serum as compared to plasma samples.
In our meta-analysis, based on studies on the diagnostic value of cCLP in RA published since 2000, we specifically extracted and listed the pre-analytical variables ‘conservation temperature’ and ‘time to centrifugation’ (Table S1). The observation that only 12 of the 18 included studies described these pre-analytics, highlights the need to sensitize researchers for awareness of these pre-analytical precautions. The matrix effect on cCLP was not evaluated as only a minority of studies were performed on plasma, which may cause bias in subgroup analysis.
Thirdly, different analytical formats are used to measure cCLP levels, and test results using different cCLP assays cannot be used interchangeably (25,36). The suspected reasons for this inter-assay variability are differences in capture and detection antibodies, measuring principle and measuring range, which all add to the variation of detecting different cCLP conformational structures. Our meta-analysis confirmed the impact of the difference in cCLP analytics on interstudy variability: in the sub-analysis of studies adhering to pre-analytical recommendations, a significant heterogeneity remained (Figure 3A). Only by omitting the studies based on the Bühlmann assay, the problem of heterogeneity was resolved. The divergence of the cCLP results obtained with the Bühlmann assay has been described earlier, contrasting the good correlation of cCLP (R&D) assays of Thermo Fisher, Werfen and Diasorin, specifically targeting the heterodimeric cCLP complex (25,36). Nevertheless, in the final sub-analysis, revealing significant study homogeneity, eight different cCLP assays were included (Figure 3B and Table S1), suggesting commutability of additional cCLP assays. More recently, a novel recombinant fusion CLP was designed, which could serve as a calibrator and additionally help in reducing the standardization problem among cCLP assays (38).
Statistically, our study had some limitations. Primarily, in order to perform the meta-analysis, we needed to recalculate median and interquartile range to mean and SD for some of the retained studies (18,28). The fact that median and interquartile range were used implies a non-normal distribution of data and thus the recalculation introduces a bias in the data set. Recalculating the data affected the effect size (i.e., the calculated mean usually tended to be a bit higher than the original median), and the amount of dispersion (i.e., false high standard deviations due to recalculation) but not the direction of the effect size. Secondly, we only included studies from 2000 onwards thus we might have missed some earlier studies. Finally, most of the included studies had small sample sizes, ranging from 25 to 969, and thus may be underpowered. However, our pooled analysis included 1,850 RA patients and 757 HC, providing more accurate data.
Conclusions
In conclusion, the inter-study variability of cCLP in RA is, besides analytical issues, mainly caused by pre-analytical confounders. Adherence to pre-analytical recommendations, significantly reduced inter-study heterogeneity and provided a reliable SMD, accurately revealing higher cCLP levels in RA versus HC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-79/rc
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-79/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-79/coif). L.V.H. has been a consultant for Thermo Fisher and has received lecture fees from Thermo Fisher. X.B. has been a consultant for Werfen and has received lecture fees from Werfen and Thermo Fisher. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. [Crossref] [PubMed]
- Tanaka Y. Rheumatoid arthritis. Inflamm Regen 2020;40:20. [Crossref] [PubMed]
- Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018;320:1360-72. [Crossref] [PubMed]
- Burgers LE, Raza K, van der Helm-van Mil AH. Window of opportunity in rheumatoid arthritis - definitions and supporting evidence: from old to new perspectives. RMD Open 2019;5:e000870. [Crossref] [PubMed]
- van Nies JA, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014;73:861-70. [Crossref] [PubMed]
- Van Hoovels L, Studenic P, Sieghart D, et al. Impact of autoimmune serology test results on RA classification and diagnosis. J Transl Autoimmun 2022;5:100142. [Crossref] [PubMed]
- Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol 2021;22:10-8. [Crossref] [PubMed]
- Deane KD, Holers VM. Rheumatoid Arthritis Pathogenesis, Prediction, and Prevention: An Emerging Paradigm Shift. Arthritis Rheumatol 2021;73:181-93. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. [Crossref] [PubMed]
- Manfredi M, Van Hoovels L, Benucci M, et al. Circulating Calprotectin (cCLP) in autoimmune diseases. Autoimmun Rev 2023;22:103295. [Crossref] [PubMed]
- Vogl T, Gharibyan AL, Morozova-Roche LA. Pro-inflammatory S100A8 and S100A9 proteins: self-assembly into multifunctional native and amyloid complexes. Int J Mol Sci 2012;13:2893-917. [Crossref] [PubMed]
- Jukic A, Bakiri L, Wagner EF, et al. Calprotectin: from biomarker to biological function. Gut 2021;70:1978-88. [Crossref] [PubMed]
- Ometto F, Friso L, Astorri D, et al. Calprotectin in rheumatic diseases. Exp Biol Med (Maywood) 2017;242:859-73. [Crossref] [PubMed]
- Carrión M, Juarranz Y, Martínez C, et al. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford) 2013;52:2177-86. [Crossref] [PubMed]
- Hammer HB, Kvien TK, Glennås A, et al. A longitudinal study of calprotectin as an inflammatory marker in patients with reactive arthritis. Clin Exp Rheumatol 1995;13:59-64. [PubMed]
- Abildtrup M, Kingsley GH, Scott DL. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol 2015;42:760-70. [Crossref] [PubMed]
- Bae SC, Lee YH. Calprotectin levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis. Postgrad Med 2017;129:531-7. [Crossref] [PubMed]
- Zeng J, Liu X, Liu J, et al. Linkage of calprotectin with inflammation, activity and treatment response of rheumatoid arthritis: a meta-analysis. Biomark Med 2022;16:1239-49. [Crossref] [PubMed]
- Hammer HB, Odegard S, Fagerhol MK, et al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis 2007;66:1093-7. [Crossref] [PubMed]
- Jonsson MK, Sundlisæter NP, Nordal HH, et al. Calprotectin as a marker of inflammation in patients with early rheumatoid arthritis. Ann Rheum Dis 2017;76:2031-7. [Crossref] [PubMed]
- Chevreau M, Paclet MH, Romand X, et al. Calprotectin is not independent from baseline erosion in predicting radiological progression in early rheumatoid arthritis. Comment on 'Calprotectin as a marker of inflammation in patients with early rheumatoid arthritis' by Jonsson et al. Ann Rheum Dis 2018;77:e84. [Crossref] [PubMed]
- Brun JG, Haga HJ, Bøe E, et al. Calprotectin in patients with rheumatoid arthritis: relation to clinical and laboratory variables of disease activity. J Rheumatol 1992;19:859-62. [PubMed]
- Chen YS, Yan W, Geczy CL, et al. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther 2009;11:R39. [Crossref] [PubMed]
- Mylemans M, Nevejan L, Van Den Bremt S, et al. Circulating calprotectin as biomarker in neutrophil-related inflammation: Pre-analytical recommendations and reference values according to sample type. Clin Chim Acta 2021;517:149-55. [Crossref] [PubMed]
- Van Hoovels L, Vander Cruyssen B, Bogaert L, et al. Pre-analytical and analytical confounders of serum calprotectin as a biomarker in rheumatoid arthritis. Clin Chem Lab Med 2019;58:40-9. [Crossref] [PubMed]
- Infantino M, Manfredi M, Albesa R, et al. Critical role of pre-analytical aspects for the measurement of circulating calprotectin in serum or plasma as a biomarker for neutrophil-related inflammation. Clin Chem Lab Med 2021;59:e317-21. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Hurnakova J, Zavada J, Hanova P, et al. Serum calprotectin (S100A8/9): an independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res Ther 2015;17:252. [Crossref] [PubMed]
- Hurnakova J, Hulejova H, Zavada J, et al. Serum calprotectin may reflect inflammatory activity in patients with active rheumatoid arthritis despite normal to low C-reactive protein. Clin Rheumatol 2018;37:2055-62. [Crossref] [PubMed]
- Gernert M, Schmalzing M, Tony HP, et al. Calprotectin (S100A8/S100A9) detects inflammatory activity in rheumatoid arthritis patients receiving tocilizumab therapy. Arthritis Res Ther 2022;24:200. [Crossref] [PubMed]
- Bettner LF, Peterson RA, Bergstedt DT, et al. Combinations of Anticyclic Citrullinated Protein Antibody, Rheumatoid Factor, and Serum Calprotectin Positivity Are Associated With the Diagnosis of Rheumatoid Arthritis Within 3 Years. ACR Open Rheumatol 2021;3:684-9. [Crossref] [PubMed]
- Korndörfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol 2007;370:887-98. [Crossref] [PubMed]
- Stephan JR, Nolan EM. Calcium-induced Tetramerization and Zinc Chelation Shield Human Calprotectin from Degradation by Host and Bacterial Extracellular Proteases. Chem Sci 2016;7:1962-75. [Crossref] [PubMed]
- Pedersen L, Birkemose E, Gils C, et al. Sample Type and Storage Conditions Affect Calprotectin Measurements in Blood. J Appl Lab Med 2018;2:851-6. [Crossref] [PubMed]
- Nevejan L, Mylemans M, Vander Cruyssen B, et al. Pre-analytical recommendations and reference values for circulating calprotectin are sample type and assay dependent. Clin Chem Lab Med 2022;60:e57-60. [PubMed]
- Nordal HH, Fagerhol MK, Halse AK, et al. Calprotectin (S100A8/A9) should preferably be measured in EDTA-plasma; results from a longitudinal study of patients with rheumatoid arthritis. Scand J Clin Lab Invest 2018;78:102-8. [Crossref] [PubMed]
- Ohmann A, Guschin D, Gerspach MA, et al. A suitable tool to globally harmonize the standardization of calprotectin, an important biomarker of autoimmune diseases. Abstract Presented at 13th International Congress on Autoimmunity 2022, Athens, Greece. 2022.
Cite this article as: Massa B, Mylemans M, Vander Cruyssen B, Infantino M, Bossuyt X, Van Hoovels L. Circulating calprotectin in rheumatoid arthritis: unravelling the impact of (pre-)analytical confounders on meta-analysis results. J Lab Precis Med 2024;9:13.



