
Genetic profile characterization of histological micropapillary and solid components in lung adenocarcinoma: a systematic review
Highlight box
Key findings
• Cases of adenocarcinoma with micropapillary component is likely to harbor epidermal growth factor receptor (EGFR) mutation, v-Raf murine sarcoma viral oncogene homolog B mutation, or ROS proto-oncogene 1 rearrangement. Cases with solid component are highly likely to be accompanied by Kirsten rat sarcoma mutation, anaplastic lymphoma kinase rearrangement, or high programmed death-ligand 1 (PD-L1) expression, and have a lower frequency of EGFR mutation.
What is known and what is new?
• Patients with micropapillary or solid components show worse prognosis even if these components are not predominant.
• Incidences of cases with micropapillary and those of solid component were found to be almost the same.
• Lung adenocarcinoma with micropapillary and solid components can be characterized using druggable genetic alternations or PD-L1 expression.
What is the implication, and what should change now?
• Micropapillary or solid component characteristics can be distinguished based on their genetic features and PD-L1 expression levels. The classification of cases with poor prognostic components can be reasonably based on the genetic profile, which can be directly related to the treatment strategy.
Introduction
Background
Micropapillary and solid components are histological features specific to adenocarcinoma in lung cancer and are morphologically identified; patients exhibiting these components show a worse prognosis. The latest International Association for the Study of Lung Cancer (IASLC) pathological classification defines the highest malignant grade (grade 3) as having 20% or higher micropapillary and/or solid patterns (1). These two components are handled equally in the pathological grading.
Rationale and knowledge gap
While numerous studies have focused on the prognostic impact of these components, only a few have explored the differences in genetic features between micropapillary and solid components. The prevalence of these cases and their genetic status have not been reviewed systematically.
Objective
The aim of this systematic review was to unveil the genetic background and refine the distinctions between these highly malignant components. We present this article in accordance with the PRISMA reporting checklist (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-88/rc).
Methods
Review process and eligibility criteria
Publications were searched through PubMed using two keywords: “lung adenocarcinoma micropapillary” or “lung adenocarcinoma solid”. A systematic review was performed according to the PRISMA guidelines (2). To determine current trends in evidence, the reviewed literature was limited to reports published in the last 5 years (publications between January 2019 and July 2023). The article type was limited to original research. There were no limitations in the inclusion criteria regarding the stage, treatment method, or methodology for genetic or protein examinations. The exclusion criteria were as follows: articles in non-English, case report(s), review, meta-analysis, letter to the editor, editorial, articles focusing on cases other than primary lung adenocarcinoma, and literature regarded as improper for this systematic review. “Books and documents”, “meta-analysis”, “review”, and “systematic review” articles were also searched using article-type filtering option in PubMed and were excluded. No other automation tool was used. Some articles published before January 2019 were included in the review via manual search or reference to citations in the literature if they were suitable for this review. Data collection was performed mainly by one author, and the decision to include the searched articles was discussed by authors.
Synthesis and assessment of results
Results are presented as median, range, and interquartile range (IQR). When an article included adenocarcinoma and other types of lung cancer, the incidence was calculated only from the adenocarcinoma data. As the review process had no limitations regarding clinical background (stage, treatment, and methodology of gene or protein detection), the appropriateness of the articles was evaluated individually. Owing to the heterogeneity of the research strategy and inclusion criteria reported in each article, a direct comparison of the reviewed literature was difficult. Results were not quantified using any unique tool, and the significance was not estimated by any statistical analysis.
This systematic review focused on the genetic background of micropapillary and solid components. Therefore, details of the results that were of little relevance to the genetic status were either described in a simple manner or omitted; the main notable results are summarized in table and figures. The possibilities of bias by searching or reporting and the certainty of outcomes are referred to in the discussion section.
Results
Systematic research and extraction of publications for review
Using two keywords for research, 1,320 and 320 candidate articles were respectively selected for review. Next, 213 and 361 articles were excluded owing to duplication and exclusion criteria, respectively. Unrelated 267 articles that included “solid” as a part of “solid tumor” or “solid appearance” as a radiological feature were also excluded. In addition, 478 articles that included little or no information on the micropapillary or solid component were excluded. After excluding duplicate articles for each keyword search, four articles were added by manually referring to the searched articles. Finally, 248 articles were reviewed (Figure 1). The reviewed articles can be mainly categorized mainly into three fields: 88, 37, and 97 articles on the prognosis, radiomics, or targetable genetic or programmed death-ligand 1 (PD-L1) features of adenocarcinoma with the micropapillary or solid components, respectively.

Distribution of micropapillary and solid adenocarcinoma
Each study included cases with a wide range of tumor-node-metastasis (TNM) stages (early and/or advanced stages) and various histological adenocarcinoma subtypes (non-invasive and/or invasive, inclusion or exclusion of variant types of adenocarcinoma; some articles included non-adenocarcinoma cases). Some studies exclusively focused on micropapillary/solid predominant cases, while others analyzed micropapillary/solid component-positive cases even if the component was not predominant. Therefore, the definitions of micropapillary or solid adenocarcinoma cases vary, and it is difficult to compare literature without bias. In the reviewed literature, on direct comparison, the prevalence of solid predominant cases was higher than that of micropapillary predominant cases in most articles [micropapillary: median 2.65% (range: 0.0–25.22%, IQR: 1.29–6.28%) in 105 publications; solid: median 11.205% (range: 0.8–50.7%, IQR: 5.19–18.83%) in 112 publications]. A comparison of the prevalence between micropapillary component-positive and solid component-positive cases revealed that the frequency of micropapillary cases increased and became similar to those of solid cases [micropapillary: median 28.91% (range: 2.39–52.48%, IQR: 16.56–44.06%) in 20 publications; solid: median 26.32% (range: 9.0–79.44%, IQR: 15.69–29.93%) in 11 publications] (Figure 2). The prevalence of IASLC grade 3 widely varied from 7.75% to 64.9% (median 20.0%, IQR: 13.5–43.1%) in 9 publications.

Clinical characteristics of cases including micropapillary and solid components
Reported cases with micropapillary or solid components show worse prognosis, even if these components were not predominant (3-5). The searched literature suggests a higher incidence of local invasion (lymphovascular invasion or spread through the air space) (6-8) or metastasis in the lymph node/brain (9-11) in micropapillary or solid component-positive cases. Regarding clinical diagnosis, the usefulness of radiomics in detecting the existence of these components has been suggested (12-15).
Genetic characteristics and PD-L1 expression in micropapillary and solid component
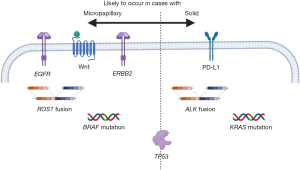
Comprehensive genetic analysis has revealed a higher tumor mutation burden (TMB) (16-18) and a higher number of mutations in tumor suppression genes (19) in cases with micropapillary or solid components. Several studies have reported a higher frequency of actionable genetic alterations; furthermore, a high incidence of epidermal growth factor receptor (EGFR) mutation (10,20-26), v-Raf murine sarcoma viral oncogene homolog B (BRAF) mutation (27-29), or ROS proto-oncogene 1 (ROS1) rearrangement (27,30,31) has been reported in adenocarcinoma with a micropapillary component. A high level of Kirsten rat sarcoma (KRAS) mutation (23,24,32-34), anaplastic lymphoma kinase (ALK) rearrangement (3,35-44), or PD-L1 expression (25,45-63) and a low frequency of EGFR mutation was suggested in adenocarcinoma with a solid component (64-66) (Table 1). One study concluded ROS1 arrangement highly occurred in solid predominant adenocarcinoma (67). Some studies have suggested that the level of these targetable alterations is related to the prognosis (21,33,54).
Table 1
| Targetable gene or PD-L1 | Expression and adenocarcinoma type | References | |
|---|---|---|---|
| Micropapillary | Solid | ||
| EGFR | High | – | (10,20-26) |
| – | Low | (64-66) | |
| KRAS | High | – | (31) |
| – | High | (23,24,32-35) | |
| ALK | High | – | (27) |
| – | High | (36-42) | |
| High | High | (3,43,44) | |
| BRAF | High | – | (27-29) |
| High | High | (16) | |
| ROS1 | High | – | (27,30,31) |
| – | High | (67) | |
| PD-L1 | – | High | (25,45-58) |
| High | High | (59-63) | |
PD-L1, programmed death-ligand 1; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma; ALK, anaplastic lymphoma kinase; BRAF, v-Raf murine sarcoma viral oncogene homolog B; ROS1, ROS proto-oncogene 1.
Other crucial targetable variants [mesenchymal-epithelial transition (MET) factor amplification (68), wingless and Int-1 (Wnt) signaling activation (16,18,69), activation or mutation of tumor protein p53 (TP53) (16,18,70-72), and high antigen Kiel 67 (Ki-67) expression (73,74)] have been suggested to occur in micropapillary or solid adenocarcinoma. An erythroblastic oncogene B-2 (ERBB2) mutation has been detected in patients with (18,75) or without (69) micropapillary component (Figure 3). Only 21 articles described the incidence of micropapillary or solid-positive cases and their relationship with genetic alternation or PD-L1 expression.
Discussion
Adenocarcinoma is the dominant histological subtype of primary lung cancer and comprises several histological components. Adenocarcinoma is also likely to harbor various types of actionable genetic alterations. The prognosis of lung adenocarcinoma largely depends on TNM stage, histological subtype, and the use of targeted therapy drugs and/or immune checkpoint inhibitors (ICIs). In the clinical setting, all cases of adenocarcinoma are classified based on the TNM staging, and resected cases are additionally subdivided based on pathological features. Genetic status and PD-L1 expression levels are essential for deciding the therapeutic strategy in adjuvant settings or recurrent cases. However, TNM stage is defined independent of pathological features, and the relationship between histological subtype and genetic status has not been sufficiently revealed. The present review reveals that micropapillary and solid components can be related to different genetic features. Although the incidence of micropapillary predominant cases is low compared with that of solid predominant cases, the frequency of cases including micropapillary components has been confirmed to be similar to that of solid component-positive cases. Recent clinical trials have shown that adjuvant targeted therapy or ICI improves outcomes in resected lung cancer cases (76,77). Patients harboring KRAS mutation or wild-type EGFR show favorable response to ICIs (77,78). Hence, genetic profiling in cases with high malignant potential is important.
The micropapillary and solid components can be characterized by different genetic features or PD-L1 expression. Several studies have suggested that genetic features and PD-L1 are highly expressed in both micropapillary and solid adenocarcinomas (3,43,44,59-63). This finding might partially be due to the heterogeneity in histology or morphological diagnosis. Some studies have included cases with micropapillary or solid components in the same cohort. These components actually often appear in the same tumor; micropapillary predominant cases can include solid components, and vice versa. Regarding diagnosis, morphological decision depends on the pathologist. The distribution of the predominant subtypes varied widely among institutions (79). This diagnostic discordance may account for the migration of genetic profiles between micropapillary and solid adenocarcinomas.
Morphology seems less important in advanced non-resected cases and is not directly related to drug indications. However, morphology is fundamental for lung cancer diagnosis and is crucial for resected cases to predict the malignant potential. Cases with equal to or more than 20% micropapillary and solid components are treated as high risk in an ongoing adjuvant osimertinib clinical trial for resected stage IA2–IA3 EGFR-mutated non-small cell lung cancer (ADAURA2) (80).
When discussing genetic alternations, the regional difference in incidence should not be ignored. EGFR mutation is more common in Asia, and the majority of the reports on EGFR mutations in our review was also from Asia. However, a previous review on the incidence of EGFR mutations by distinguishing between Asian and non-Asian cohorts showed that the incidence of EGFR mutation was higher in micropapillary component-predominant cases even in non-Asians (81). The incidence of EGFR mutation seems to be higher in micropapillary cases regardless of geographic differences. The evidence regarding the relationship between the prevalence of PD-L1 expression or druggable genetic alterations and histological features needs to be gathered, particularly in cases with micropapillary or solid component.
This systematic review has some limitations. First, we only utilized PubMed as a source; despite the size and popularity of PubMed, the use of several sources is warranted. Second, although we searched articles published within 5 years to identify current trends in evidence, the methodology, sensitivity, and accuracy to detect the genetic alternations were quite different among studies. In addition, several studies did not primarily focus on the genetic background of the micropapillary and solid components, and genetic status or incidence data were obtained as secondary information from each article. The terms “high” and “low” regarding incidence are not absolutely quantified results; rather, they represent the conclusion of each reviewed article. The impact of results was not compared among studies, and the significance of genetic status was not analyzed statistically; thus, the heterogeneity, sensitivity, certainty, and risk of bias stemming from missing results in each study could not be evaluated in the synthesized data. Further reviews unifying patient background and performing statistical analysis are warranted. Third, we used simple search keywords to include a large selection of candidate studies. However, bias was inevitable because the information about the prevalence of adenocarcinoma subtypes and clinically targetable genetic alternations are generally included in studies on lung cancer, and evaluating articles not identified by simple keyword searches might not be practical.
Conclusions
Patients with micropapillary and solid components showed worse prognosis. They are handled equally for pathological grading, and the incidence of micropapillary- or solid-positive cases is similar. These two components can be distinguished based on their genetic features or PD-L1 expression level. Classification of cases with these poor prognostic components can be reasonably based on the genetic profile, which can indicate the prognostic status and is directly related to the treatment strategy.
Acknowledgments
We would like to thank Editage (https://www.editage.jp/) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-88/rc
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-88/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-88/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moreira AL, Ocampo PSS, Xia Y, et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol 2020;15:1599-610. [Crossref] [PubMed]
- PRISMA statement. Available online: https://www.prisma-statement.org/
- Wang Y, Yang X, Liu B, et al. Percentage of Newly Proposed High-Grade Patterns Is Associated with Prognosis of Pathological T1-2N0M0 Lung Adenocarcinoma. Ann Surg Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Hou Y, Song W, Chen M, et al. The presence of lepidic and micropapillary/solid pathological patterns as minor components has prognostic value in patients with intermediate-grade invasive lung adenocarcinoma. Transl Lung Cancer Res 2022;11:64-74. [Crossref] [PubMed]
- Zhai W, Liang D, Duan F, et al. Prognostic Nomograms Based on Ground Glass Opacity and Subtype of Lung Adenocarcinoma for Patients with Pathological Stage IA Lung Adenocarcinoma. Front Cell Dev Biol 2021;9:769881. [Crossref] [PubMed]
- Choi SH, Jeong JY, Lee SY, et al. Clinical implication of minimal presence of solid or micropapillary subtype in early-stage lung adenocarcinoma. Thorac Cancer 2021;12:235-44. [Crossref] [PubMed]
- Song T, Jiang L, Zhuo Z, et al. Impacts of thoracoscopic surgery and high grade histologic subtypes on spread through air spaces in small stage I lung adenocarcinomas. J Cancer Res Clin Oncol 2019;145:2375-82. [Crossref] [PubMed]
- Zombori T, Sejben A, Tiszlavicz L, et al. Architectural Grade Combined With Spread Through Air Spaces (STAS) Predicts Recurrence and is Suitable for Stratifying Patients Who Might Be Eligible for Lung Sparing Surgery for Stage I Adenocarcinomas. Pathol Oncol Res 2020;26:2451-8. [Crossref] [PubMed]
- Song CY, Kimura D, Sakai T, et al. Novel approach for predicting occult lymph node metastasis in peripheral clinical stage I lung adenocarcinoma. J Thorac Dis 2019;11:1410-20. [Crossref] [PubMed]
- Wang K, Xue M, Qiu J, et al. Genomics Analysis and Nomogram Risk Prediction of Occult Lymph Node Metastasis in Non-Predominant Micropapillary Component of Lung Adenocarcinoma Measuring ≤ 3 cm. Front Oncol 2022;12:945997. [Crossref] [PubMed]
- Li C, Shen Y, Hu F, et al. Micropapillary pattern is associated with the development of brain metastases and the reduction of survival time in EGFR-mutation lung adenocarcinoma patients with surgery. Lung Cancer 2020;141:72-7. [Crossref] [PubMed]
- He B, Song Y, Wang L, et al. A machine learning-based prediction of the micropapillary/solid growth pattern in invasive lung adenocarcinoma with radiomics. Transl Lung Cancer Res 2021;10:955-64. [Crossref] [PubMed]
- Ding H, Xia W, Zhang L, et al. CT-Based Deep Learning Model for Invasiveness Classification and Micropapillary Pattern Prediction Within Lung Adenocarcinoma. Front Oncol 2020;10:1186. [Crossref] [PubMed]
- Park S, Lee SM, Noh HN, et al. Differentiation of predominant subtypes of lung adenocarcinoma using a quantitative radiomics approach on CT. Eur Radiol 2020;30:4883-92. [Crossref] [PubMed]
- Shao D, Su F, Zou X, et al. Pixel-Level Classification of Five Histologic Patterns of Lung Adenocarcinoma. Anal Chem 2023;95:2664-70. [Crossref] [PubMed]
- Caso R, Sanchez-Vega F, Tan KS, et al. The Underlying Tumor Genomics of Predominant Histologic Subtypes in Lung Adenocarcinoma. J Thorac Oncol 2020;15:1844-56. [Crossref] [PubMed]
- Talvitie EM, Vilhonen H, Kurki S, et al. High tumor mutation burden predicts favorable outcome among patients with aggressive histological subtypes of lung adenocarcinoma: A population-based single-institution study. Neoplasia 2020;22:333-42. [Crossref] [PubMed]
- Meng F, Zhang Y, Wang S, et al. Whole-Exome Sequencing Reveals the Genomic Features of the Micropapillary Component in Ground-Glass Opacities. Cancers (Basel) 2022;14:4165. [Crossref] [PubMed]
- Zhang Y, Ma Y, Li Y, et al. Comparative analysis of co-occurring mutations of specific tumor suppressor genes in lung adenocarcinoma between Asian and Caucasian populations. J Cancer Res Clin Oncol 2019;145:747-57. [Crossref] [PubMed]
- Zhang J, Sun J, Zhang Z, et al. Driver mutation profiles and clinicopathological correlation in pulmonary adenocarcinoma with a micropapillary component. Hum Pathol 2019;85:242-50. [Crossref] [PubMed]
- Kishi N, Ito M, Miyata Y, et al. Intense Expression of EGFR L858R Characterizes the Micropapillary Component and L858R Is Associated with the Risk of Recurrence in pN0M0 Lung Adenocarcinoma with the Micropapillary Component. Ann Surg Oncol 2020;27:945-55. [Crossref] [PubMed]
- Fu Y, Zha J, Wu Q, et al. Stromal micropapillary pattern and CD44s expression predict worse outcome in lung adenocarcinomas with micropapillary pattern. Pathol Res Pract 2023;248:154595. [Crossref] [PubMed]
- Saito R, Ninomiya H, Okumura S, et al. Novel Histologic Classification of Small Tumor Cell Nests for Lung Adenocarcinoma With Prognostic and Etiological Significance: Small Solid Nests and Pure Micropapillary Nests. Am J Surg Pathol 2021;45:604-15. [Crossref] [PubMed]
- Ding Y, Zhang L, Guo L, et al. Comparative study on the mutational profile of adenocarcinoma and squamous cell carcinoma predominant histologic subtypes in Chinese non-small cell lung cancer patients. Thorac Cancer 2020;11:103-12. [Crossref] [PubMed]
- Cai Y, Wu H, Shi X, et al. Heterogeneous components of lung adenocarcinomas confer distinct EGFR mutation and PD-L1 expression. BMC Cancer 2020;20:148. [Crossref] [PubMed]
- Suda K, Murakami I, Yu H, et al. Heterogeneity of EGFR Aberrations and Correlation with Histological Structures: Analyses of Therapy-Naive Isogenic Lung Cancer Lesions with EGFR Mutation. J Thorac Oncol 2016;11:1711-7. [Crossref] [PubMed]
- Li D, Ding L, Ran W, et al. Status of 10 targeted genes of non-small cell lung cancer in eastern China: A study of 884 patients based on NGS in a single institution. Thorac Cancer 2020;11:2580-9. [Crossref] [PubMed]
- Ahn HY, Lee CH, Lee MK, et al. BRAF V600E Mutation of Non-Small Cell Lung Cancer in Korean Patients. Medicina (Kaunas) 2023;59:1085. [Crossref] [PubMed]
- Gow CH, Hsieh MS, Lin YT, et al. Validation of Immunohistochemistry for the Detection of BRAF V600E-Mutated Lung Adenocarcinomas. Cancers (Basel) 2019;11:866. [Crossref] [PubMed]
- Zhang X, Jiang Y, Yu H, et al. A comprehensive study on the oncogenic mutation and molecular pathology in Chinese lung adenocarcinoma patients. World J Surg Oncol 2020;18:172. [Crossref] [PubMed]
- Kang H, Lv H, Tung TH, et al. EGFR co-mutation is associated with the risk of recurrence in invasive lung adenocarcinoma with the micropapillary component. Asian J Surg 2024;47:201-7. [Crossref] [PubMed]
- Cao H, Ma Z, Li Y, et al. Prognostic value of KRAS G12C mutation in lung adenocarcinoma stratified by stages and radiological features. J Thorac Cardiovasc Surg 2023;166:e479-99. [Crossref] [PubMed]
- Ma Z, Zhang Y, Deng C, et al. The prognostic value of Kirsten rat sarcoma viral oncogene homolog mutations in resected lung adenocarcinoma differs according to clinical features. J Thorac Cardiovasc Surg 2022;163:e73-85. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]
- Rekhtman N, Ang DC, Riely GJ, et al. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol 2013;26:1307-19. [Crossref] [PubMed]
- Li P, Gao Q, Jiang X, et al. Comparison of Clinicopathological Features and Prognosis between ALK Rearrangements and EGFR Mutations in Surgically Resected Early-stage Lung Adenocarcinoma. J Cancer 2019;10:61-71. [Crossref] [PubMed]
- Zhao R, Zhang J, Han Y, et al. Clinicopathological Features of ALK Expression in 9889 Cases of Non-small-Cell Lung Cancer and Genomic Rearrangements Identified by Capture-Based Next-Generation Sequencing: A Chinese Retrospective Analysis. Mol Diagn Ther 2019;23:395-405. [Crossref] [PubMed]
- Lee HK, Kwon MJ, Seo J, et al. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in ALK-positive lung cancer: Comparison with EGFR-mutated lung cancer. Pathol Res Pract 2019;215:459-65. [Crossref] [PubMed]
- Liu Y, Ye X, Yu Y, et al. Prognostic significance of anaplastic lymphoma kinase rearrangement in patients with completely resected lung adenocarcinoma. J Thorac Dis 2019;11:4258-70. [Crossref] [PubMed]
- Choi Y, Kim KH, Jeong BH, et al. Clinicoradiopathological features and prognosis according to genomic alterations in patients with resected lung adenocarcinoma. J Thorac Dis 2020;12:5357-68. [Crossref] [PubMed]
- Han X, Fan J, Li Y, et al. Value of CT features for predicting EGFR mutations and ALK positivity in patients with lung adenocarcinoma. Sci Rep 2021;11:5679. [Crossref] [PubMed]
- Jiang Z, Li C, Lu H. Next generation sequencing detection in archival surgically resected lung adenocarcinoma specimens harbouring the anaplastic lymphoma kinase fusion protein. J Pak Med Assoc 2021;71:531-6. [PubMed]
- Song L, Zhu Z, Wu H, et al. Individualized nomogram for predicting ALK rearrangement status in lung adenocarcinoma patients. Eur Radiol 2021;31:2034-47. [Crossref] [PubMed]
- Fujibayashi Y, Tane S, Kitazume M, et al. Resected stage I anaplastic lymphoma kinase-positive lung adenocarcinoma has a negative impact on recurrence-free survival. Thorac Cancer 2022;13:1109-16. [Crossref] [PubMed]
- Zito Marino F, Rossi G, Montella M, et al. Heterogeneity of PD-L1 Expression in Lung Mixed Adenocarcinomas and Adenosquamous Carcinomas. Am J Surg Pathol 2020;44:378-86. [Crossref] [PubMed]
- Song P, Wu S, Zhang L, et al. Correlation Between PD-L1 Expression and Clinicopathologic Features in 404 Patients with Lung Adenocarcinoma. Interdiscip Sci 2019;11:258-65. [Crossref] [PubMed]
- Naso JR, Wang G, Pender A, et al. Intratumoral heterogeneity in programmed death-ligand 1 immunoreactivity is associated with variation in non-small cell lung carcinoma histotype. Histopathology 2020;76:394-403. [Crossref] [PubMed]
- Lee SE, Kim YJ, Sung M, et al. Association with PD-L1 Expression and Clinicopathological Features in 1000 Lung Cancers: A Large Single-Institution Study of Surgically Resected Lung Cancers with a High Prevalence of EGFR Mutation. Int J Mol Sci 2019;20:4794. [Crossref] [PubMed]
- Wu J, Sun W, Wang H, et al. The correlation and overlaps between PD-L1 expression and classical genomic aberrations in Chinese lung adenocarcinoma patients: a single center case series. Cancer Biol Med 2019;16:811-21. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Endo M, et al. Programmed death ligand 1 protein expression is positively correlated with the solid predominant subtype, high MIB-1 labeling index, and p53 expression and negatively correlated with epidermal growth factor receptor mutations in lung adenocarcinoma. Hum Pathol 2021;108:12-21. [Crossref] [PubMed]
- Jain E, Sharma S, Aggarwal A, et al. PD-L1 expression and its clinicopathologic and genomic correlation in the non-small cell lung carcinoma patients: An Indian perspective. Pathol Res Pract 2021;228:153497. [Crossref] [PubMed]
- Cruz-Rico G, Avilés-Salas A, Popa-Navarro X, et al. Association of Lung Adenocarcinoma Subtypes According to the IASLC/ATS/ERS Classification and Programmed Cell Death Ligand 1 (PD-L1) Expression in Tumor Cells. Pathol Oncol Res 2021;27:597499. [Crossref] [PubMed]
- Simundza I, Krnic D, Juricic J, et al. Expression of PD-L1 Is Associated with Inflammatory Microenvironment in Surgical Specimens of Non-Small Cell Lung Cancer. J Pers Med 2021;11:767. [Crossref] [PubMed]
- Takamochi K, Hara K, Hayashi T, et al. Programmed death-ligand 1 expression and its associations with clinicopathological features, prognosis, and driver oncogene alterations in surgically resected lung adenocarcinoma. Lung Cancer 2021;161:163-70. [Crossref] [PubMed]
- Zhang D, Gao X, Yan Z, et al. Heterogeneous distribution of PD-L1 expression in the IASLC/ATS/ERS classification of lung adenocarcinoma. Int J Clin Oncol 2022;27:105-11. [Crossref] [PubMed]
- Miyazawa T, Morikawa K, Otsubo K, et al. Solid histological component of adenocarcinoma might play an important role in PD-L1 expression of lung adenocarcinoma. Thorac Cancer 2022;13:24-30. [Crossref] [PubMed]
- Kojima K, Sakamoto T, Kasai T, et al. A quantitative evaluation of the histological type dependence of the programmed death-ligand 1 expression in non-small cell lung cancer including various adenocarcinoma subtypes: a cross-sectional study. Jpn J Clin Oncol 2022;52:281-5. [Crossref] [PubMed]
- Miyazawa T, Marushima H, Saji H, et al. PD-L1 Expression in Non-Small-Cell Lung Cancer Including Various Adenocarcinoma Subtypes. Ann Thorac Cardiovasc Surg 2019;25:1-9. [Crossref] [PubMed]
- Forest F, Casteillo F, Da Cruz V, et al. Heterogeneity of PD-L1 expression in lung adenocarcinoma metastasis is related to histopathological subtypes. Lung Cancer 2021;155:1-9. [Crossref] [PubMed]
- Tancoš V, Grendár M, Farkašová A, et al. Programmed death ligand 1 protein expression, histological tumour differentiation and intratumoural heterogeneity in pulmonary adenocarcinoma. Pathology 2020;52:538-45. [Crossref] [PubMed]
- Bulutay P, Firat P, Zeren EH, et al. The importance of histological patterns on PD-L1 staining heterogeneity: Should we use pattern-based approach for selecting tumor samples for PD-L1 testing in lung adenocarcinomas? Turk J Med Sci 2021;51:204-13. [Crossref] [PubMed]
- Zheng Q, Huang Y, Zeng X, et al. Clinicopathological and molecular characteristics associated with PD-L1 expression in non-small cell lung cancer: a large-scale, multi-center, real-world study in China. J Cancer Res Clin Oncol 2021;147:1547-56. [Crossref] [PubMed]
- Zhou J, Lin H, Ni Z, et al. Expression of PD-L1 through evolution phase from pre-invasive to invasive lung adenocarcinoma. BMC Pulm Med 2023;23:18. [Crossref] [PubMed]
- Boukansa S, Benbrahim Z, Gamrani S, et al. Correlation of Epidermal Growth Factor Receptor Mutation With Major Histologic Subtype of Lung Adenocarcinoma According to IASLC/ATS/ERS Classification. Cancer Control 2022;29:10732748221084930. [Crossref] [PubMed]
- Osawa J, Shimada Y, Maehara S, et al. Clinical usefulness of the 3-tier classification according to the proportion of morphological patterns for patients with pathological stage I invasive lung adenocarcinoma. Gen Thorac Cardiovasc Surg 2021;69:943-9. [Crossref] [PubMed]
- Yu W, Zhao Q, Xia C, et al. Validation of stage groupings in the eighth edition of the tumor node metastasis classification for lung adenocarcinoma. Thorac Cancer 2019;10:483-91.
- Xu Y, Chang H, Wu L, et al. High prevalence of ROS1 gene rearrangement detected by FISH in EGFR and ALK negative lung adenocarcinoma. Exp Mol Pathol 2020;117:104548. [Crossref] [PubMed]
- Overbeck TR, Cron DA, Schmitz K, et al. Top-level MET gene copy number gain defines a subtype of poorly differentiated pulmonary adenocarcinomas with poor prognosis. Transl Lung Cancer Res 2020;9:603-16. [Crossref] [PubMed]
- Zhu L, Yang S, Zheng L, et al. WNT/β-catenin pathway activation via Wnt1 overexpression and Axin1 downregulation correlates with cadherin-catenin complex disruption and increased lymph node involvement in micropapillary-predominant lung adenocarcinoma. J Thorac Dis 2020;12:5906-15. [Crossref] [PubMed]
- Li P, Liu L, Wang D, et al. Genomic and clinicopathological features of lung adenocarcinomas with micropapillary component. Front Oncol 2022;12:989349. [Crossref] [PubMed]
- Ahn B, Yoon S, Kim D, et al. Clinicopathologic and genomic features of high-grade pattern and their subclasses in lung adenocarcinoma. Lung Cancer 2022;170:176-84. [Crossref] [PubMed]
- Ashok Kumar P, Karimi M, Basnet A, et al. Association of Molecular Profiles and Mutational Status With Distinct Histological Lung Adenocarcinoma Subtypes. An Analysis of the LACE-Bio Data. Clin Lung Cancer 2023;24:528-40. [Crossref] [PubMed]
- Tanaka R, Fujiwara M, Sakamoto N, et al. Cytomorphometric and flow cytometric analyses using liquid-based cytology materials in subtypes of lung adenocarcinoma. Diagn Cytopathol 2022;50:394-403. [Crossref] [PubMed]
- Li Z, Li F, Pan C, et al. Tumor cell proliferation (Ki-67) expression and its prognostic significance in histological subtypes of lung adenocarcinoma. Lung Cancer 2021;154:69-75. [Crossref] [PubMed]
- Hayashi T, Kohsaka S, Takamochi K, et al. Histological characteristics of lung adenocarcinoma with uncommon actionable alterations: special emphasis on MET exon 14 skipping alterations. Histopathology 2021;78:987-99. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Tsutani Y, Goldman JW, Dacic S, et al. Adjuvant Osimertinib vs. Placebo in Completely Resected Stage IA2-IA3 EGFR-Mutated NSCLC: ADAURA2. Clin Lung Cancer 2023;24:376-80. [Crossref] [PubMed]
- Clay TD, Russell PA, Do H, et al. Associations between the IASLC/ATS/ERS lung adenocarcinoma classification and EGFR and KRAS mutations. Pathology 2016;48:17-24. [Crossref] [PubMed]
Cite this article as: Ito M, Suda K, Tsutani Y. Genetic profile characterization of histological micropapillary and solid components in lung adenocarcinoma: a systematic review. J Lab Precis Med 2024;9:26.


