
Editorial on “Cerebrospinal fluid total protein reference intervals derived from 20 years of patient data”
Analysis of cerebrospinal fluid (CSF) proteins continues being a basic tool for diagnosis and follow up of many neurological diseases, even when at present some consider that advanced imaging techniques have displaced CSF studies. In certain clinical settings, CSF-total proteins (TP) is sometimes the closest approximation to the central nervous system (CNS), and diagnostic or referral decisions with only CSF TP might be necessary.
The main fraction of proteins in the normal CSF originates from blood, approximately 20% are predominantly brain-derived, although rarely brain-specific. The same as in blood, albumin is the most concentrated protein, representing 35–80% of total CSF protein. All blood-derived CSF proteins can be synthesized in peripheral organs and in the CNS, with the exception of albumin which is only synthesized in the liver. Hence, being the origin of CSF albumin exclusively from blood, it’s CSF/serum concentration quotient is an ideal representation of the dynamics of blood derived proteins, which are source-related and modified by CSF flow rate, offering information regarding the function of the blood-CSF barrier (1).
Elevated CSF total protein is highly suggestive of neurological disease. Increased TP in the CSF can be related to: (I) increased entry of plasma proteins due to increased permeability of the blood-CSF barrier; (II) local synthesis of proteins within the CNS, being the clinical interest mainly focused to IgG; and (III) impaired resorption of CSF proteins by the arachnoid villi. The most frequent cause of high CSF TP in clinical settings is increased albumin, which points to a damaged blood-CSF barrier, thus TP concentration can guide the clinician in future steps to take in the management of neurological patients, when other more specific CSF techniques are not available (2).
In this context, the work published by McCudden et al. (3) encloses a great importance. As these authors state, although CSF TP concentration is widely used in the differential diagnosis of neurological disorders, and the increase of CSF TP concentration with age is extensively recognized, sex-partitioned reference intervals are rarely applied to make effective clinical decisions due to the lack of appropriate reference intervals. Their study based on a 20-year hospital-based analysis of more than 6,000 CSF samples showed a marked dependence of CSF-TP with age, suggesting that different reference intervals based on age would be more accurate than a single cutoff value. They reported that the reference cutoff of 0.45 g/L is near the 97.5th percentile protein value for subjects in the 18–25 years old reference interval; thereby overestimating the frequency of abnormal CSF-TP values over this range of age, and even more so with increasing age.
Additionally, significant differences were reported by these authors between males and females (nearly 0.06 g/L), which is consistent with previous reports (4,5), with a similar difference between genders (0.04 g/L). McCudden et al., suggest that endocrine differences and muscle mass could influence in the CSF-TP sex-difference reported, as in other biochemical measures (3), although verification of this hypothesis was not explored.
An important issue in this paper was that during the study period, 3 different automatic-instruments were employed (Siemens Vista, Beckman Lx20 and Roche 917), and although a modest difference was observed among them, reference intervals partitioned by instrument/method did not show substantial differences (95% CIs overlapped independently of the instrument). This is a particularly relevant finding which supports that even when employing different CSF TP quantification methods and instruments, the general results are very similar.
McCudden’s article presents a methodologically well-designed study, with multiple strengths, such as: the large sample size, the extensive exclusion criteria employed to select patients, and the analysis of different automatic instruments and measurement methods along the 20-year period, as possible sources of bias.
It is important to take two issues into consideration: first, autoimmunity is a frequent etiological factor in neurological diseases, showing a clear female prevalence; and second, the frequency of several brain disorders is highly age-dependant. McCudden’s study confirms with a very large sample size and rigorous mathematical calculations, what other authors have previously reported. Therefore, the age and sex-partitioned reference intervals for CSF-TP and the overestimation of altered values with age, defined by McCudden et al., are very important findings that should be considered by clinicians in the management of neurological diseases.
To compare the results obtained at the Institute of Neurology and Neurosurgery, we conducted a retrospective survey of the CSFs studied during a ten-year period [2008–2017] and included only those CSF samples with normal electrophoretic patterns by polyacrylamide gel electrophoresis. CSF TP was determined employing Lowry’s spectrophotometric method (6). Fifty-nine children (57.6% females; aged 2 months – 15 years) and 871 adults (65.8% females; aged 16–86 years) were included (total CSF samples =930). Protein concentration was significantly lower in children with respect to adults (33.7±10.4 vs. 41.4±9.5 mg/dL; t=−5.242, P=0.0000). For adults higher TP were observed in men with respect to women (43.6±9.3 vs. 40.2±9.4 respectively; t=−5.153, P=0.0000), while no difference was observed according to gender in children. TP concentration was significantly different when different age groups were evaluated, with a clear tendency towards higher TP levels with increasing age (Figure 1).
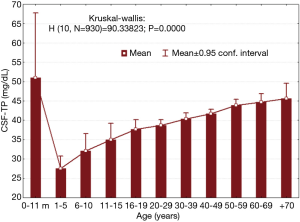
The 97.5th value for the 20−29 years old age group was 53.8 mg/dL, which is higher than that reported by McCudden et al. (3), but is closer to that reported by Hegen et al. (50 mg/dL) (7). Although higher CSF-TP mean concentration was observed in our study, in general our results are very similar. It must be kept in mind that the methods for protein analysis are different and that possibly some altered CSFs could have been included in our group of patients. Notwithstanding, the age dependent increase (with the exception of infants younger than 11 months) is very obvious.
In a previous investigation we had conducted (8), 155 normal CSFs were obtained from subjects without a history of neurological diseases: 109 children (where meningoencephalitis diagnosis was suspected, but later discarded) and 55 adults (undergoing orthopedic surgery with spinal anesthesia). CSF TP levels for the age intervals studied in subjects over 15 years of age showed no statistical difference between them, but lower CSF TP in children as compared to adults was evidenced. Although this investigation had the strength of studying the CSF of neurologically normal persons, the limitation of a much smaller sample size must be considered. McCudden et al.’s article did not include children, nevertheless, it is necessary to emphasize the importance of age-related reference values in this population too.
Kahlmann et al. recently published a study including 6,145 CSF samples collected from 3,623 children aged 6 months to 18 years, based on the analysis of samples using the Bhattacharya method, a standard procedure conducted by clinical chemists to establish reference values based on previously acquired diagnostic data. The results were further confirmed in a subgroup of 378 children with a diagnosis not usually associated with increased CSF TP concentration. Their findings were in line with other studies reporting lower CSF total protein levels in children than in adults, the lowest values were observed between the age of 2 and 6 years with a gradual increase in CSF total protein level from 6 to 18 years. In children under the age of 6 months they observed the highest TP levels and the highest variation (9). Very high CSF TP concentration was also reported recently in a large cohort of neonates (n=3,557; 127 mg/dL) and in infants from 29 to 60 days of age (n=4,209; 99 mg/dL) (10), as well as in 218 neonates with normal CSF white blood cell counts: 89±37 mg/dL (11). The cause of lower protein levels in children has been related to a higher turnover of CSF than in adults, which may increase the ability to clear proteins from the CSF (9).
Concluding, the investigation carried out by McCudden et al. offers very important information concerning differentiated age and gender related reference values for CSF TP, which have a great relevance in making effective clinical decisions in the management of neurological patients, when other more specific CSF techniques are not available. These reference values should be preferably established by each laboratory employing different techniques, amongst which the Bhattacharya method based on previously acquired diagnostic data seems to be the most accessible. Nevertheless, if this possibility is not available, the overlap of CSF TP reference intervals obtained with most of the techniques in use, gives us reason to believe that the reference intervals presented by these authors could be a much more reliable cutoff than the widespread cutoff of 45 mg/dL. On the other hand we would like to emphasize the importance of considering age-specific reference intervals for children too.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Baohong Yue (Hematology lab in Dept. of Clinical Laboratory Medicine, The First Affiliated Hospital, College of Medicine, Zhengzhou University, Zhengzhou, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.03.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci 2003;21:79-96. [PubMed]
- Regeniter A, Kuhle J, Mehling M, et al. A modern approach to CSF analysis: pathophysiology, clinical application, proof of concept and laboratory reporting. Clin Neurol Neurosurg 2009;111:313-8. [Crossref] [PubMed]
- McCudden CR, Brooks J, Figurado P, et al. Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. Clin Chem 2017;63:1856-65. [Crossref] [PubMed]
- Mertin J, Wisser H, Doerr P. Studies on the normal concentration range of total protein and the total protein fractions of the cerebrospinal fluid by electrophoresis on cellulose acetate (author's transl). Z Klin Chem Klin Biochem 1971;9:337-40. [PubMed]
- Breebaart K, Becker H, Jongebloed FA. Investigation of reference values of components of cerebrospinal fluid. J Clin Chem Clin Biochem 1978;16:561-5. [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75. [PubMed]
- Hegen H, Auer M, Zeileis A, et al. Upper reference limits for cerebrospinal fluid total protein and albumin quotient based on a large cohort of control patients: implications for increased clinical specificity. Clin Chem Lab Med 2016;54:285-92. [Crossref] [PubMed]
- González-Quevedo Monteagudo A. Electroforesis de proteínas del líquido cefalorraquídeo normal en niños y adultos. Rev Mex Neuroci 2008;9:242-7.
- Kahlmann V, Roodbol J, van Leeuwen N, et al. Validated age-specific reference values for CSF total protein levels in children. Eur J Paediatr Neurol 2017;21:654-60. [Crossref] [PubMed]
- Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal Fluid Reference Values for Young Infants Undergoing Lumbar Puncture. Pediatrics 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Noureldein M, Mardare R, Pickard J, et al. Cerebrospinal fluid protein and glucose levels in neonates with a systemic inflammatory response without meningitis. Fluids Barriers CNS 2018;15:8. [Crossref] [PubMed]
Cite this article as: González-Quevedo A, Sánchez MP, González García S. Editorial on “Cerebrospinal fluid total protein reference intervals derived from 20 years of patient data”. J Lab Precis Med 2018;3:35.


